Test: Electronic Structure - MCAT MCQ
10 Questions MCQ Test - Test: Electronic Structure
Suppose that an electron starts in the n=4 shell of a neutral hydrogen atom. How many photons will be emitted once it has fallen to the n=1 shell?
Based on electronic structure, which of the following ions would be expected to be pulled toward a magnet?
| 1 Crore+ students have signed up on EduRev. Have you? Download the App |
Photons of the same wavelength strike four metal targets in a vacuum. Electrons are measured leaving the surfaces at various speeds. Based on the velocities of the ejected electrons, which metal has the largest work function?
Which of the following is the electronic structure of N?
Which of the following is the electronic structure of Fe2+?
Suppose that four photons each hit a hydrogen atom and raise an electron from an initial orbit, n1, to a final orbit, n2, Which photon had the shortest wavelength?
What are the quantum numbers describing the highest angular momentum number, spin-up electron in a neutral, unexcited chlorine atom’s highest-energy orbital?
If you know that an electron is inside a 25 cm wide X-ray radiography machine what is the error on the most precise measurement that you could make of its momentum? Planck’s constant is 6.6 X 10−34 m2, kg/s.
Electrons in four hydrogen atoms fall from an excited state to the ground state, where the quantum number n=1, giving off a single photon in the process. Based on the photon, which electron was the farthest distance away from the nucleus before it fell to it ground state (h = 6.6 X 10−34 m2 X kg/s is Planck’s constant)?
Which of the following quantum number sets indicates the lowest energy orbital of an electron in a neutral aluminum atom?


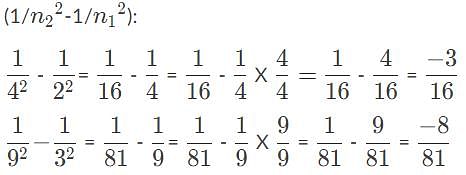
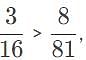 , so the photon that drives the electron from n1 = 2, n2 = 4, has the higher energy and shorter wavelength.
, so the photon that drives the electron from n1 = 2, n2 = 4, has the higher energy and shorter wavelength.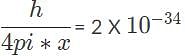 kg x m/s, the most precise measurement for the electron’s momentum when it is inside the radiography machine.
kg x m/s, the most precise measurement for the electron’s momentum when it is inside the radiography machine.














