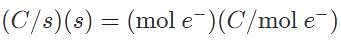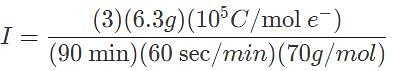Test: Electrochemisrty - MCAT MCQ
10 Questions MCQ Test - Test: Electrochemisrty
Given the table of reduction potentials, which metals will displace hydrogen gas out of acid?

I. Aluminum
II. Palladium
III. Potassium
IV. Zinc

II. Palladium
III. Potassium
IV. Zinc
The salt bridge is essential in maintaining electrical neutrality in a Daniell cell. In the absence of a salt bridge, the two compartments will accumulate the opposite charges and prevent further reaction. For the salt bridge to function properly, there must be an appropriate electrolyte. Which of the following statements most accurately explains whether an electrolyte is appropriate for the salt bridge in the following electrochemical cell?
| 1 Crore+ students have signed up on EduRev. Have you? Download the App |
Which of the following statements accurately describes the difference between galvanic and electrolytic cells?
An electrolytic cell has three main components, an electrolyte and two electrodes (an anode and a cathode) and the electrolyte can come in two forms, either as molten or in an aqueous solution. Which of the following statements accurately describes an electrolytic cell?
A current is passed through a Ga(NO3)3 for 1.5 hours, and after this time period the mass of metal produced was 6.3 grams. What is the current, in amperes, that is required to produce such an amount of gallium?
Which of the following is an example of a spontaneous redox reaction?
Which of the following is an example of a non-spontaneous redox reaction?
Which of the following factors affects the rate of a chemical reaction in an electrochemical cell?
MCQ: Which of the following is the standard electrode potential for the hydrogen electrode?