NEET Exam > NEET Tests > Test: Nature of Matter & Its Properties (April 17) - NEET MCQ
Test: Nature of Matter & Its Properties (April 17) - NEET MCQ
Test Description
10 Questions MCQ Test - Test: Nature of Matter & Its Properties (April 17)
Test: Nature of Matter & Its Properties (April 17) for NEET 2024 is part of NEET preparation. The Test: Nature of Matter & Its Properties (April 17) questions and answers have been prepared
according to the NEET exam syllabus.The Test: Nature of Matter & Its Properties (April 17) MCQs are made for NEET 2024 Exam.
Find important definitions, questions, notes, meanings, examples, exercises, MCQs and online tests for Test: Nature of Matter & Its Properties (April 17) below.
Solutions of Test: Nature of Matter & Its Properties (April 17) questions in English are available as part of our course for NEET & Test: Nature of Matter & Its Properties (April 17) solutions in
Hindi for NEET course.
Download more important topics, notes, lectures and mock test series for NEET Exam by signing up for free. Attempt Test: Nature of Matter & Its Properties (April 17) | 10 questions in 20 minutes | Mock test for NEET preparation | Free important questions MCQ to study for NEET Exam | Download free PDF with solutions
Test: Nature of Matter & Its Properties (April 17) - Question 1
Few quantities with their units are listed below. Mark the units which are not correctly matched.
(i) Density: kg m-3
(ii) Velocity of light: m s-1
(iii) Planck's constant: J-1 s-1
(iv) Acceleration: m s-2
(v) Force: kg m
(i) Density: kg m-3
(ii) Velocity of light: m s-1
(iii) Planck's constant: J-1 s-1
(iv) Acceleration: m s-2
(v) Force: kg m
Detailed Solution for Test: Nature of Matter & Its Properties (April 17) - Question 1
Test: Nature of Matter & Its Properties (April 17) - Question 2
Match the prefixes present in column I with their multiples in column II and mark the appropriate choice.
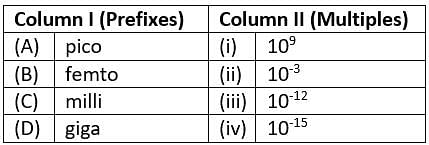

Detailed Solution for Test: Nature of Matter & Its Properties (April 17) - Question 2
| 1 Crore+ students have signed up on EduRev. Have you? Download the App |
Test: Nature of Matter & Its Properties (April 17) - Question 3
Choose the correct statement about I, II and III.
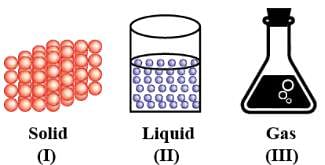

Detailed Solution for Test: Nature of Matter & Its Properties (April 17) - Question 3
Test: Nature of Matter & Its Properties (April 17) - Question 4
Mark the conversion factor which is not correct.
Detailed Solution for Test: Nature of Matter & Its Properties (April 17) - Question 4
Test: Nature of Matter & Its Properties (April 17) - Question 5
Consider the following figure,
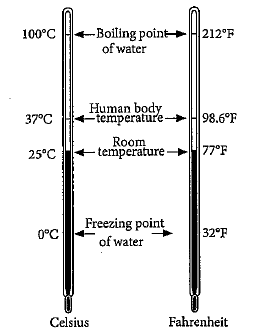
The correct relationship between fahrenheit and celsius scale is:
Detailed Solution for Test: Nature of Matter & Its Properties (April 17) - Question 5
Test: Nature of Matter & Its Properties (April 17) - Question 6
Which of the following is an element?
Detailed Solution for Test: Nature of Matter & Its Properties (April 17) - Question 6
Test: Nature of Matter & Its Properties (April 17) - Question 7
Which of the following is a compound?
Detailed Solution for Test: Nature of Matter & Its Properties (April 17) - Question 7
Detailed Solution for Test: Nature of Matter & Its Properties (April 17) - Question 8
Test: Nature of Matter & Its Properties (April 17) - Question 9
The correct relationship between picometer and nanometer is
Detailed Solution for Test: Nature of Matter & Its Properties (April 17) - Question 9
Test: Nature of Matter & Its Properties (April 17) - Question 10
Which one of the following is not a mixture?
Detailed Solution for Test: Nature of Matter & Its Properties (April 17) - Question 10
Information about Test: Nature of Matter & Its Properties (April 17) Page
In this test you can find the Exam questions for Test: Nature of Matter & Its Properties (April 17) solved & explained in the simplest way possible.
Besides giving Questions and answers for Test: Nature of Matter & Its Properties (April 17), EduRev gives you an ample number of Online tests for practice
Download as PDF

















