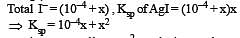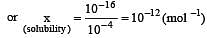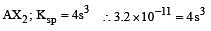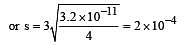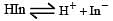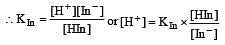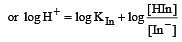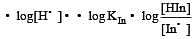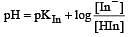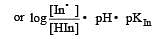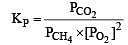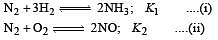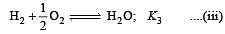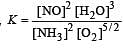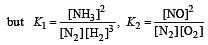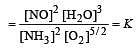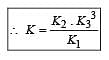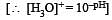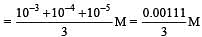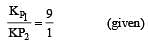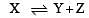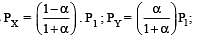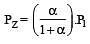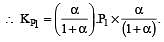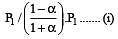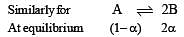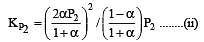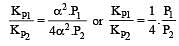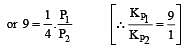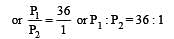Test: Equllibrium (September 18) - NEET MCQ
15 Questions MCQ Test - Test: Equllibrium (September 18)
Solubility of MX2-type eletrolytes is 0.5 × 10–4 mole/lit, then find out Ksp of electrolytes [2002]
Solution of 0.1 N NH4OH and 0.1 N NH4Cl has pH 9.25. Then find out pKb of NH4OH [2002]
| 1 Crore+ students have signed up on EduRev. Have you? Download the App |
The reaction quotient (Q) for the reaction
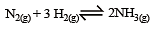
is given by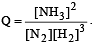
The reaction will proceed from right to left if [2003]
where Kc is the equilibrium constant
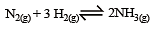
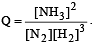
Which one of the following orders of acid strength is correct? [2003]
The solubility product of AgI at 25ºC is 1.0 × 10–16 mol2 L–2. The solubiliy of AgI in 10–4 N solution of KI at 25ºC is approximately(in mol L–1) [2003]
The solubility product of a sparingly soluble salt AX2 is 3.2 x 10-11 . Its solubility ( in moles/litre) is
[2004]
The rapid change of pH near the stoichiometric point of an acid-base titration is the basis of indicator detection. pH of the solution is related to ratio of the concentrations of the conjugate acid (HIn) and base (In–) forms of the indicator by the expression [2004]
What is the correct relation ship between the pHs of isomolar solutions of sodium oxide (pH1), sodium sulphide (pH2), sodium selenide (pH3) and sodium telluride (pH4)? [2 00 5]
For the reaction [2006]
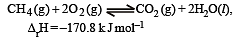
Which of the following statements is not true ?
The hydrogen ion concentration of a 10–8 M HCl aqueous solution at 298 K (Kw = 10–14) is [2006]
Which of the following pairs constitutes a buffer?
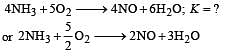
The following equilibrium constants are given:
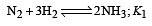
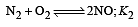
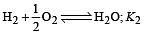
The equilibrium constant for the oxidation of NH3 by oxygen to give NO is
Equal volumes of three acid solutions of pH 3, 4 and 5 are mixed in a vessel. What will be the H+ ion concentration in the mixture ? [2008]
Equimolar solutions of the following were prepared in water separately. Which one of the solutions will record the highest pH ? [2008]
The values of Kp1 and Kp2 for the reactions
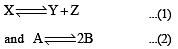
are in the ratio of 9 : 1. If degree of dissociation of X and A be equal, then total pressure at equilibrium (1) and (2) are in the ratio :


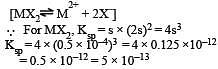
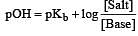

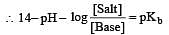
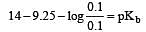
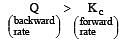 i.e the reaction will be fast in backward direction i.e rb > rf.
i.e the reaction will be fast in backward direction i.e rb > rf.