Chemical Bonding And Molecular Structure Practice Test (1) - Class 9 MCQ
25 Questions MCQ Test - Chemical Bonding And Molecular Structure Practice Test (1)
Octet of electrons, represents a particularly stable electronic arrangement.. Atoms achieve the stable octet when they are linked by chemical bonds. This rule is associated with one of the following theories
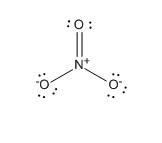
Structures of CO2−3CO32−. ion given below explains the phenomenon of in terms of
| 1 Crore+ students have signed up on EduRev. Have you? Download the App |
The maximum number of hydrogen bonds that a molecule of water can have is
For a stable molecule the value of bond order must be
Polarity in a molecule and hence the dipole moment depends primarily on electronegativity of the constituent atoms and shape of a molecule. Which of the following has the highest dipole moment?
Inertness of noble gas was observed to be due to their electronic configurations: Choose the most appropriate
The product of the magnitude of the charge and the distance between the centres of positive and negative charge.
During the formation of a chemical bond
In acetylene molecule, between the carbon atoms there are
The types of hybrid orbitals of nitrogen in No+2, No−3 and NH+4 respectively are expected to be
Stable outer octet of electrons is achieved in chlorine atom during the formation of NaCl by:
the valence shell electron pair repulsion (vsepr) theory helps in the
Which of the following has maximum number of lone pairs associated with Xe?
In PO3−4 ion the formal charge on the oxygen atom of P–O bond is
Isostructural species are those which have the same shape and hybridisation. Among the given species identify the isostructural pairs.
The bond formed, as a result of the electrostatic attraction between the positive and negative ions was termed as
In the formation of hydrogen molecule, overlapping of atomic orbitals which results in the pairing of electrons. These are:
The structure of IF7is
In which of the following substances will hydrogen bond be strongest?
In NO−3 ion, the number of bond pairs and lone pairs of electrons on nitrogen atom are
The number of dots around the Lewis symbols for the elements represent
In NO−3 ion, the number of bond pairs and lone pairs of electrons on nitrogen atom are
The number of types of bonds between two carbon atoms in calcium carbide is
When a gas phase atom in its ground state gains an electron. This is called
Amongst the following elements whose electronic configurations are given below, the one having the highest ionisation enthalpy is