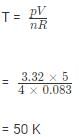P. Bahadur Test: States of Matter (Old NCERT) - NEET MCQ
25 Questions MCQ Test - P. Bahadur Test: States of Matter (Old NCERT)
The average Kinetic energy and Thermal energy are proportional to the:
Above the Critical temperature (TC) of carbon dioxide:
| 1 Crore+ students have signed up on EduRev. Have you? Download the App |
A student forgot to add the reaction mixture to the round bottomed flask at 27 °C but instead he/she placed the flask on the flame. After a lapse of time, he realized his mistake, and using a pyrometer he found the temperature of the flask was 477 °C. What fraction of air would have been expelled out?
The pressure of a 1:4 mixture of dihydrogen and dioxygen enclosed in a vessel is one atmosphere. What would be the partial pressure of dioxygen?
The ease with which the electron cloud of a particle can be distorted is called its
Gases have much lower density than the solids and liquids because
The three states of matter of H2O are in equilibrium at
Calculate the temperature of 4.0 mol of a gas occupying 5 dm3 at 3.32 bar. (R = 0.083 bar dm3K−1mol−1)
As the temperature increases, average kinetic energy of molecules increases. What would be the effect of increase of temperature on pressure provided the volume is constant?
Molecules in the interior of liquid experience intermolecular
Charles’ law states that pressure remaining constant, the volume of a fixed mass of a gas is
Surface tension decreases as the temperature is raised because
Calculate the total number of electrons present in 1.4 g of dinitrogen gas.
Gases possess characteristic critical temperature which depends upon the magnitude of intermolecular forces between the particles. Following are the critical temperatures of some gases. From the data what would be the order of liquefaction of these gases? Start writing the order from the gas liquefying first
Gay Lussac’s law states that at constant volume, pressure of a fixed amount of a gas
The van der Waals Equation adjusts the measured volume
Capillarity results from a competition between the
The statements for laws of chemical combinations are given below. Mark the statement which is not correct.
Water has high surface tension and high capillarity because of
The van der Waals Equation adjusts the measured pressure
Atmospheric pressures recorded in different cities are as follows: Consider the above data and mark the place at which liquid will boil first.
Which curve in Figure represents the curve of ideal gas?