Equilibrium - Practice Test (1) - Class 9 MCQ
25 Questions MCQ Test - Equilibrium - Practice Test (1)
When the rate of evaporation is equal to the rate of condensation, it is called
the concentrations in an equilibrium mixture are related by the following equilibrium equation, 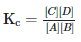 Where Kc is called as
Where Kc is called as
| 1 Crore+ students have signed up on EduRev. Have you? Download the App |
Which of the following is not a general characteristic of equilibria involving physical processes?
At 500 K, equilibrium constant, Kc, for the following reaction is What would be the equilibrium constant Kc for the reaction 2HI (g) ⇌⇌ H2(g) +I2(g)
0.023 g of sodium metal is reacted with 100 cm3 of water. The pH of the resulting solution is ______.
In the dynamic equilibrium stage, one of the following events take place. Choose the right one.
Equilibrium constant for the reaction, 4NH3(g) + 5O2(g)!4NO(g) + 6H2O (g) is written as
For the reaction N2O4(g) ⇌⇌2NO2(g), the value of K is 50 at 400 K and 1700 at 500 K. Which of the following option is incorrect?
H2S is passed into one dm3 of a solution containing 0.1 mole of Zn2+ and 0.01 mole of Cu2+ till the sulphide ion concentration reaches 8.1 × 10−−19 moles. Which one of the following statements is true? [Kspof ZnS and CuS are 3 × 10−−22and 8 × 10−−36respectively]
the rates of transfer of molecules from ice into water and of reverse transfer from water into ice are equal at atmospheric pressure and 273 K. Both the processes occur simultaneously and at the same rate so that the amount of ice and water remains constant. This process is called
For reactions involving gases, however, it is usually more convenient to express the equilibrium constant in terms of
At a particular temperature and atmospheric pressure, the solid and liquid phases of a pure substance can exist in equilibrium. Which of the following term defines this temperature?
pH value of which one of the following is NOT equal to one?
H2O(l) ⇌ H2O (vap). This equation best illustrates the
If the Kc is neither too small nor too large, one of the following will happen
When hydrochloric acid is added to cobalt nitrate solution at room temperature, the following reaction takes place and the reaction mixture becomes blue. On cooling the mixture it becomes pink. On the basis of this information mark the correct answer.
The ionisation of hydrochloric in water is given below:
A vessel at 1000 K contains CO2 with a pressure of 0.5 atm. Some of the CO2 is converted into CO on the addition of graphite. If the total pressure at equilibrium is 0.8 atm, the value of K is
During the process of transformation from liquid to vapour, the pressure exerted by the water molecules at a given temperature remains constant. This is called
Find KcKc for the following reaction, if CO2CO2pressure is in atmospheres:
The pH of neutral water at 25∘C is 7.0. As the temperature increases, ionisation of water increases, however, the concentration of H+ ions and OH− ions are equal. What will be the pH of pure water at 60∘C?
The aqueous solution of sugar does not conduct electricity. However, when sodium chloride is added to water, it conducts electricity. How will you explain this statement on the basis of ionisation and how is it affected by concentration of sodium chloride?
What is the best description of the change that occurs when Na2O(s) is dissolved in water?

















