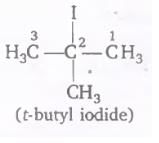Haloalkanes And Haloarenes Practice Quiz - 1 - Class 9 MCQ
25 Questions MCQ Test - Haloalkanes And Haloarenes Practice Quiz - 1
The IUPAC name for tertiary butyl iodide is
Upon oxidation, one of the following halogen containing compounds forms an extremely poisonous gas – – – + O2→2COCl2 + 2HCl
| 1 Crore+ students have signed up on EduRev. Have you? Download the App |
Necessary conditions for any system to be aromatic:
A dibromo derivative of an alkane reacts with sodium metal to form an alicyclic hydrocarbon. The derivative is
Give IUPAC names of the following compound:
Carbon – halogen bond of alkyl halides is responsible for their nucleophilic substitution, elimination and their reaction with metal atoms to form organometallic compounds because of their
The synthesis of 3 – octyne is achieved by adding a bromoalkane into a mixture of sodium amide and an alkyne. The bromoalkane and alkyne respectively are
The position of –Br in the compound in CH3CH==CHC(Br)(CH3)2 can be classified as
Which one of the following is not a chiral molecule?
Toluene reacts with a halogen in the presence of iron (III) chloride giving ortho and para halo compounds. The reaction is
A dibromo derivative of an alkane reacts with sodium metal to form an alicyclic hydrocarbon. The derivative is ______.
The reaction RX + 2Na + RX → R – R + 2NaX is called?
A mixture containing two enantiomers in equal proportions
p, p’ – Dichlorodiphenyltrichloroethane is a
Decomposition of benzene diozonium chloride by using Cu2Cl2/HCl to form chlorobenzene is
The following
(CH3)2CHCH2CH2Cl
(CH3)2CHCH(Cl)CH3
(CH3)2C(Cl)CH2CH3
CH3CH(CH2Cl)CH2CH3
are the possible structural isomers expected to be formed if one of the hydrogen atoms is replaced by chlorination. The original compound is
In the reaction Pent – 2 – ene, 2 – Bromopentane, Pent – 1 – ene 2 – Bromopentane on heating with alcoholic KOH, forms two compounds: Pent – 1 – ene and Pent – 2 – ene. Which one of the following statements is true
Tetrachloromethane (Carbon tetrachloride)is
Write structures of the following compounds:
(i) 4-tert. Butyl-3-iodoheptane
(ii) 1,4-Dibromobut-2-ene
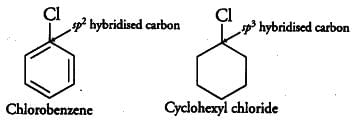
Explain why the dipole moment of chlorobenzene is lower than that of cyclohexyl chloride?
Maximum number of molecules of CH3I that can react with a molecule of CH3NH2 is ?
For the same alkyl group, an alkyl iodide has a higher boiling point than alkyl fluoride because,