Test: Hydrogen (Old NCERT) - JEE MCQ
25 Questions MCQ Test - Test: Hydrogen (Old NCERT)
In the earth’s atmosphere, hydrogen exists in the form of
presence of extensive hydrogen bonding between water molecules leads to
| 1 Crore+ students have signed up on EduRev. Have you? Download the App |
Temporary hardness It can be removed in boiling by precipitating
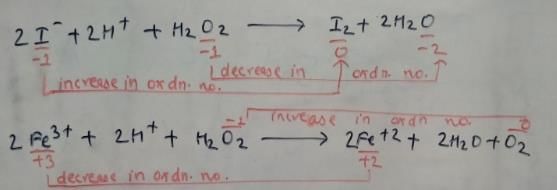
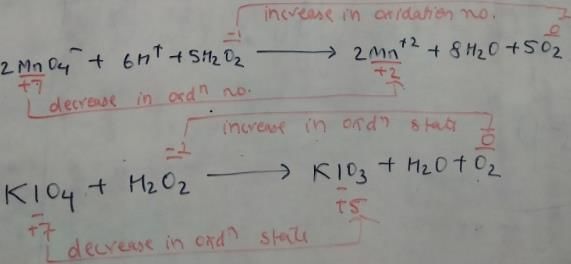
Which of the following equations depict the oxidising nature of H2O2?
For moderation of the climate and body temperature of living beings, the responsible factor is:
In Calgon’s method, one of the following chemical is used to remove hardness of water
Which of the following equation depicts reducing nature of H2O2?
Approximately what percent of matter in the universe is believed to consist of hydrogen?
In the gas phase water is a bent molecule with a bond angle of
The three isotopes of hydrogen in terms of chemical properties show one of the following properties
Stoichiometric compounds of dihydrogen are formed with
Consider the reactions
(A) H2O2 + 2HI → I2 +2H2O
(B) HOCl + H2O2 → H3O + Cl - - + O2
Which of the following statements is correct about H2O2 with reference to these reactions? Hydrogen perioxide is ________.
Which of the following statements are not true for hydrogen?
Dihydrogen under certain reaction conditions, combines with almost all elements to form binary compounds, called hydrides except with few which are given below Choose one of the options
How many hydrogen-bonded water molecule(s) are associated in CuSO4 . 5H2O ?
Dihydrogen can be prepared on commercial scale by different methods. In its preparation by the action of steam on hydrocarbons, a mixture of CO and H2 gas is formed. It is known as ____________.
Atoms like N, O and F in hydrides have lower boiling points than those of the subsequent group member hydrides. It is because of