Organic Chemistry : Some Basic Principles - Practice Test (1) - Class 9 MCQ
25 Questions MCQ Test - Organic Chemistry : Some Basic Principles - Practice Test (1)
Hybridisation in methane (CH4), ethene (C2H4), ethyne (C2H2) involves s and p orbitals. Choose the correct hybrid orbitals in the options given below for CH4, C2H4CH4, C2H4 and, C2H4 respectively
Correct IUPAC name the following compound is
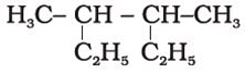
| 1 Crore+ students have signed up on EduRev. Have you? Download the App |
In which of the following, functional group isomerism is not possible?
The technique that is extensively used to separate mixtures into their components, purify compounds and also to test the purity of compounds is
Electrophiles are electron seeking species. Which of the following groups contain only electrophiles?
(i)
(ii)
(iii)
(iv)
Change in hybridisation affects the carbon’s and the organic compound’s
Benzene and other related ring compounds (benzenoid) such as given below are called:
The fragrance of flowers is due to the presence of some steam volatile organic compounds called essential oils. These are generally insoluble in water at room temperature but are miscible with water vapour in vapour phase. A suitable method for the extraction of these oils from the flowers is:
Aniline is separated by technique from aniline . water mixture
In the four compounds below, which of the following pairs are position isomers? (i) I and II (ii) II and III(iii) II and IV(iv) III and IV
Condense the following complete structural formula given below. Choose the appropriate answer given below
The following compound is called
During hearing of a court case, the judge suspected that some changes in the documents had been carried out. He asked the forensic department to check the ink used at two different places. According to you which technique can give the best results?
Sodium cyanide, sulphide and halide,( -CN, -S and -H, coming from organic compound) so formed on sodium fusion are extracted from the fused mass by boiling it with distilled water. This is called
In Carius method, the organic compound is heated with
2-bromo butane can be represented in various ways. Choose the incorrect one from the following
Compounds contain carbon atoms joined in the form of a ring are called
The principle involved in paper chromatography is
Geometrical isomers and Optical isomers are:
Write the state of hybridisation of carbon in the compound, HC≡≡N and shapes of each of the molecules
Which of the following is the correct IUPAC name?
The molecular formula C3H8O represents two alcohols: propan-1-ol and propan-2-ol. This property is called
In which of the following compounds the carbon marked with asterisk is expected to have greatest positive charge?
When an organic compound is present in an aqueous medium, it is separated by
In sulphur estimation, 0.157 g of an organic compound gave 0.4813 g of barium sulphate. What is the percentage of sulphur in the compound?

















