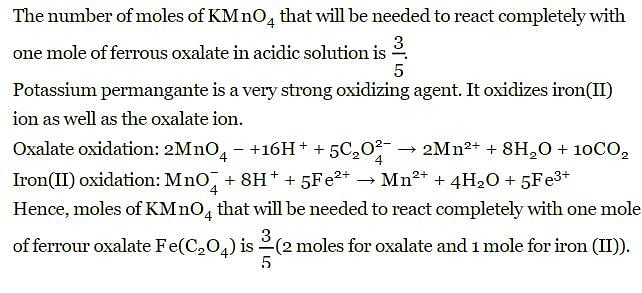Test: Redox reactions & Equivalent in Terms of Redox Reactions (August 27) - NEET MCQ
10 Questions MCQ Test - Test: Redox reactions & Equivalent in Terms of Redox Reactions (August 27)
The decomposition of hydrogen peroxide to form water and oxygen is an example of
Oxidation number denotes the oxidation state of an element in a compound ascertained on the basis that electron in a covalent bond belongs
| 1 Crore+ students have signed up on EduRev. Have you? Download the App |
Identify the correct statements with reference to the given reaction 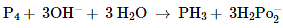
The highest value of oxidation number changes from 1 to 7
The more positive the value of E0, the greater is the tendency of the species to get reduced. Using the standard electrode potential of redox couples given below find out which of the following is the strongest oxidising agent.
E0values : Fe3 + / Fe2+ = +0.77; I2(s)/l- = +0.54; cu2+/ Cu = +0.34; Ag+ / Ag = +0.80V
Consider conversion of MnCI2 into
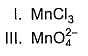
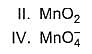
Increasing order of equivalent weights of MnCI2 in these conversions is
The number of moles of KMnO4 that will be needed to react with one mole of ferrous oxalate Fe(C2O4) in acidic solution is
Photosynthesis of carbohydrates in plants takes place as
6CO2 +12H2O  C6H12O6 + 6O2 + 6H2O
C6H12O6 + 6O2 + 6H2O
Equivalent weight of CO2 and C6H12O6 respectively are
Equivalent w eight of KMnO4 and its reduced species in different mediums are given
In which of the following reactions, equivalent mass of the underlined is equal to molar mass?