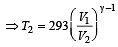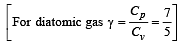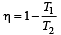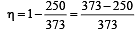Test: Thermodynamics (October 10) - NEET MCQ
10 Questions MCQ Test - Test: Thermodynamics (October 10)
First law of thermodynamics is consequence of conservation of [1988]
A thermodynamic process is shown in the figure.
The pressures and volumes corresponding to some points in the figure are
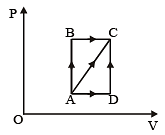
PA = 3 × 104 Pa
VA = 2 × 10-3 m3
PB = 8 × 104 Pa
VD = 5 × 10–3 m3.
In process AB, 600 J of heat is added to the system and in process BC, 200 J of heat is added to the system. The change in internal energy of the system in process AC would be [1991]
The pressures and volumes corresponding to some points in the figure are

VA = 2 × 10-3 m3
PB = 8 × 104 Pa
VD = 5 × 10–3 m3.
In process AB, 600 J of heat is added to the system and in process BC, 200 J of heat is added to the system. The change in internal energy of the system in process AC would be [1991]
| 1 Crore+ students have signed up on EduRev. Have you? Download the App |
If for a gas,  , the gas is made up of molecules which are [1992]
, the gas is made up of molecules which are [1992]
 , the gas is made up of molecules which are [1992]
, the gas is made up of molecules which are [1992]An ideal gas A and a real gas B have their volumes increased from V to 2V under isothermal conditions. The increase in internal energy [1993]
Which of the following is not thermodynamical function ? [1993]
An ideal carnot engine, whose efficiency is 40% receives heat at 500 K. If its efficiency is 50%, then the intake temperature for the same exhaust temperature is [1995]
An ideal gas undergoing adiabatic change has the following pressure-temperature relationship [1996]
A diatomic gas initially at 18ºC is compressed adiabatically to one eighth of its original volume.The temperature after compression will be [1996]
The efficiency of a Carn ot engine operating between the temperatures of 100ºC and –23ºC will be
[1997]
We consider a thermodynamic system. If ΔU represents the increase in its internal energy and W the work done by the system, which of the following statements is true? [1998]


 hence gas is monoatomic.
hence gas is monoatomic.


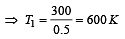
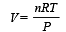 ....(2)
....(2) constant
constant