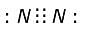Test: Types of Bond and Fajan Rule (May 21) - NEET MCQ
10 Questions MCQ Test - Test: Types of Bond and Fajan Rule (May 21)
The electronic configuration of four atoms are given in brackets:
L(1s22s22p1);
M(1s22s22p5);
Q(1s22s22p63s1);
R(1s22s22p2);
The element that would most readily form a diatomic molecule is
M(1s22s22p5);
Q(1s22s22p63s1);
R(1s22s22p2);
How many number of electrons are involved in the formation of a nitrogen molecule?
| 1 Crore+ students have signed up on EduRev. Have you? Download the App |
Lewis structure of HCI, H2O and NH3 are
Which of the following is electron deficient?
Considering x-axis as the internuclear axis, which out of the following will not form sigma bond.
Which of these molecules have non-bonding electron pairs on the central atom?
I. SF4
II. ICI3
III. SO2
IV. NH3
V. SiF4
Different kinds of bonds and interaction present within CuSO4 • 5H2O. They can be
I. σ-bond
II. π-bond
III. coordinate bond
IV. electrostatic force of attraction
V. H-bond due to dipole-dipole interaction
VI. H-bond due to ion-dipole interaction
Select the correct types of bonds/interactions.
Arrange NaCI, MgCI2, AICI3, SiCI4 in increasing solubility in ether (non-polar solvent).
Aqueous solution of a mixture contains LiCI, CuCI, NaCI and AICI3. This is shaken with ether. What is/are left in water?