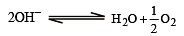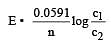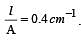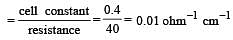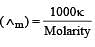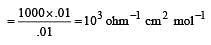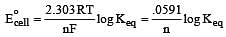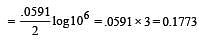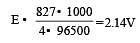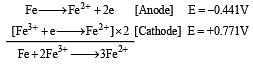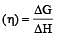Test: Electrochemistry (November 16) - NEET MCQ
15 Questions MCQ Test - Test: Electrochemistry (November 16)
On electrolysis of dilute sulphuric acid using platinum electrodes, the product obtained at the anode will be [1992]
The standard reduction poten tials at 25°C of
Li+/ Li, Ba 2+ / Ba, Na+ / Na
and Mg 2+ / Mg are – 3.03, – 2.73, – 2.71 and – 2.37 volt respectively. Which one of the following is the strongest oxidising agent? [1994]
Li+/ Li, Ba 2+ / Ba, Na+ / Na
and Mg 2+ / Mg are – 3.03, – 2.73, – 2.71 and – 2.37 volt respectively. Which one of the following is the strongest oxidising agent? [1994]
| 1 Crore+ students have signed up on EduRev. Have you? Download the App |
The most durable metal plating on iron to protect against corrosion is [1994]
An electr och emical cell is set up as: Pt; H2 (1atm)|HCl(0.1 M) || CH3COOH (0.1 M)| H2 (1atm); Pt. The e.m.f of this cell will not be zero, because [1995]
If 0.01 M solution of an electrolyte has a resistance of 40 ohms in a cell having a cell constant of 0.4 cm–1, then its molar conductance in ohm–1 cm2 mol–1 is [1997]
Standard potentials (Eº) for some half-reactions are given below :
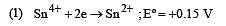
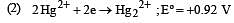
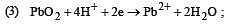 Eº = +1.45 V
Eº = +1.45 V
Based on the above, which one of the following statements is correct ? [1997]
Without losing its concentration ZnCl2 solution cannot be kept in contact with [1998]
What is the Eºcell for the reaction
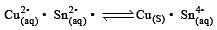
at 25ºC if the equilibrium constant for the reaction is 1 × 106? [1999]
Specific conductance of a 0.1 N KCl solution at 23ºC is 0.012 ohm–1 cm–1. Resistance of cell containing the solution at same temperature was found to be 55 ohm. The cell constant is [2000]
The most convenient method to protect the bottom of ship made of iron is [2001]
In electrolysis of NaCl when Pt electrode is taken then H2 is liberated at cathode while with Hg cathode it forms sodium amalgam. This is because [2002]
On the basis of the information available from the reaction
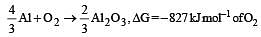
the minimum e.m.f required to carry out an electrolysis of Al2O3 is (F = 96500 C mol–1)
If 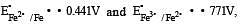 , the standard EMF of the reaction Fe + 2Fe3+ → 3Fe2+ will be [2006]
, the standard EMF of the reaction Fe + 2Fe3+ → 3Fe2+ will be [2006]
The efficiency of a fuel cell is given by [2007]