Test: Integrated Rate Equations (November 22) - NEET MCQ
10 Questions MCQ Test - Test: Integrated Rate Equations (November 22)
The half life of a first order reaction is equal to:
For zero order reaction, linear plot was obtained for [A] vs t, the slope of the line is equal to:
| 1 Crore+ students have signed up on EduRev. Have you? Download the App |
The first order rate constant for the decomposition of N2O5 is 6.2 x 10-4 sec-1. The t1/2 of the decomposition reaction is
Find the overall order of a reaction whose rate constant is k = 3x10-4 s-1
Which of the following represents the expression for the 3/4th life of a first order reaction?
The type of reaction that gives constant half life is
The half life period of first order reaction is 15 min. Its rate constant will be equal to
For zero order reaction, the integrated rate equation is:
The half life of a zero order reaction is equal to:
If [A] is the concentration of reactant A at any time t and [Ao] is the concentration at t=0, then for the first order reaction the rate equation can be written as:



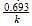 , where k is the rate constant.
, where k is the rate constant.














