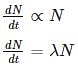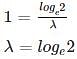Test: CSIR-NET Chemical Sciences Mock Test - 3 - UGC NET MCQ
30 Questions MCQ Test - Test: CSIR-NET Chemical Sciences Mock Test - 3
In an examination 10,000 students appeared. The result revealed the number of students who have:
- Passed in all five subjects = 5583
- Passed in three subjects only = 1400
- Passed in two subjects only = 1200
- Passed in one subject only = 735
- Failed in English only = 75
- Failed in Physics only = 145
- Failed in Chemistry only = 140
- Failed in Mathematics only = 200
- Failed in Bio-science only = 157
The number of students passed in at least four subjects is:
Which of the following is not a method of a survey?
| 1 Crore+ students have signed up on EduRev. Have you? Download the App |
Brinjal plant belongs to the family ________.
The mean of the ages of father and his son is 27 years. After 18 years, father will be twice as old as his son. Their present ages are
The stone formed in human kidney consist mostly of _______ .
The measures of the three angles of a triangle are in the ratio 3:4:5. What is the measure of the biggest angle?
Given are the differences between a thesis and a research article. Choose the correct option.
(a) Thesis covers all the aspects of a research study and research article covers one idea or gist of entire research in brief.
(b) A thesis will have an extensive presentation of methods but research article will have a controlled presentation of specific methodology.
(c) Thesis is a general requirement for an academic degree while research article may or may not be required for getting a degree.
(d) Thesis has an exhaustive list of references whereas research article will have a selective list only.
(e) Thesis takes a longer time to complete but article needs less time.
A tropical location B is 20 km N15° E of A. Another location C is N45°W of A and S75° W of B. How far (in km) is C from A?
(N15° E denotes a direction 15° east of north.)
A substance decays at a rate proportional to the amount of the substance itself. If half of the substance decays in one year, then what is the proportionality constant?
If 25 is written as
11001 (1 × 24 +1 × 23 + 0 × 22 + 0 × 21 +1 × 20),
then how will 101 be written?
Suppose A=1, B=2, C=3, D=4,...,X=24, Y=25, Z=26.
What is the value of 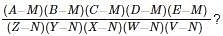
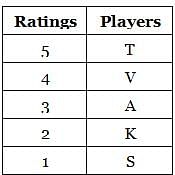
Suppose the ratings of five players A, V, T, K and S are all distinct. The average of S and A’s ratings is equal to the minimum of the ratings of V and K. T has a higher rating than A, and A’s rating is equal to the average of V and K’s ratings. Who has the third highest rating among the five players?
In a hall, the fraction of people seated is 9/13 and the fraction of chairs occupied is 7/9. If the number of empty chairs is 36, how many more chairs are needed to seat everyone in the hall?
A, B, C and D are consecutive integers such that B2 - A2 = 103 Then D2 - C2 =
The qualifying marks for an exam attempted by a large number of students are set at half of the median of the marks scored in that exam. Under this condition, 10% of the candidates fail. If the qualifying marks are set at the median of the scores, and if no two students have the same marks, the percentage of candidates failing will be closest to
How much nitrogen must be added to a 20% nitrogen solution to obtain 100 Kg of 40% nitrogen solution?
A process is carried out for the feed mixture of 95% air and 5% ammonia vapor, the compressor recovered 40% of ammonia, what is the exit pressure of the compressor, if the partial pressure of ammonia vapor is 10 mm Hg?
A reactor is supplied with a feed of composition 46% C3H7O4, 44% O2 and 10% N2, if 50% of the limiting reagent is converted into product, what is the percentage of O2 in the product?
The specific volume of SO2 is 10 m3/Kg, at 5 Pa, H = 100 J/Kg and at 10 Pa, H = 250 J/Kg, what is the heat transferred to the system?
An unsteady-state system, with 10 liters as initial amount of water in the vessel, water flow in rate is 8 liters/ s and flow out rate is 5 liters/s, what will be the amount of water in the vessel after 10 seconds?
What is the final amount of fluid in the vessel if the system is in steady state?
A reaction has produced 5 moles of water vapor and 10 liter bone dry air at 27ºC and 15 atm, what is the humidity?
What is the specific gravity of a substance with density 100 kg/m3 with respect to reference substance of density 100 lb/m3?
Condensation starts at which point?
How long would it take for an irreversible process to complete?
A closed U-tube manometer has a gas on one end with pressure 5 Pa and other end has vacuum, what is the approximate height difference of the liquid if the density of liquid is 10 Kg/m3?
A container has gas mixture of 20% H2 and 80% N2 with total pressure P, now same amount of O2 is added to the mixture, what is the new partial pressure of N2?
Which of the following is used to measure a pressure of only gas?
In air conditioning process, what has to be done first to the moist air?
An unsteady state system, the flow in rate of A is 5 mole/s, what is the flow out rate of B if the accumulation was 10 mole in 5 seconds?