Revisal Problems (Past 13 Years) JEE Main (Structure Of Atom) - JEE MCQ
24 Questions MCQ Test - Revisal Problems (Past 13 Years) JEE Main (Structure Of Atom)
Direction (Q. Nos. 1-24) This section contains 24 multiple choice questions. Each question has four choices (a), (b), (c) and (d), out of which ONLY ONE option is correct.
Q. Wavelength associated with water molecule moving with a velocity of 500 ms-1 is
Assume H-atom (rH = 0.0529nm) and proton (rp =1.5x 10-15m)to be spherical. Fraction of the space in an atom of hydrogen that is occupied by the nucleus is
| 1 Crore+ students have signed up on EduRev. Have you? Download the App |
What electronic transition in a hydrogen atom, starting from the orbit n = 7 will produce infrared light of wavelength 2170 nm?
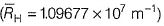
If for a given value of n, perimissible values of azimuthal quantum number l be 0,1,2...... n, then magnetic moment of chromium (24) would be
Velocity of the electron in H-atom in second orbit is
If uncertainties in the measurements of position and momentum are equal, then uncertainty in the measurement of velocity is
For the two moving particles A and B, following values are given
Q. Ratio of de-Broglie wavelength associated with the particles A and B is
The wave number of the spectral line in the emission spectrum of H-atom will be equal to 8/9 times the Rydberg’s constant if the electron jumps from
Which one of the following constitutes a group of the isoelectronic species?
In an experiment reproducing the measurements of Rutherford and his co-workers, 22 x10-3 mg of He gas was collected in one year from a sample of radium. This sample was observed to emit 1. 06 x 1011 α - particlesper second. Thus, Avogadro’s number is
Medical experts generally consider a lead level of 30 μg Pb per (dL) of blood to pose a significant health risk (Pb =208). Number of lead atoms per cm3 of blood is
In Schrodinger wave equation given below
Which hydrogen like species will have same radius as that of Bohr’s orbit of hydrogen atom?
An atom for which the electron probability distribution is spherically symmetric about the nucleus is
Which of the following statements in relation to the hydrogen atom is correct?
Which of the following options does not represent ground state EC of an atom?
Statement I :The electronic configuration of N -atom is represented as :
Statement II : The electronic configuration of the ground state of an atom is the one which has the greatest multiplicity.
If n and l are respectively the principal and azimuthal quantum numbers, then the expression for calculating the total number of electrons in any energy level is
3d-suborbit first occurs in fourth orbit. It has
How fast is an electron moving if it has a wavelength equal to the distance it travels in one second?
In the presence of magnetic field, d-suborbit is
Electrons are used to determine the structure of crystal surfaces. To have diffraction, the wavelength λ, of the electrons should be of the order of the lattice constant, which is typically 0.30 nm. What energy do such electrons have?
The number of d-electrons in Fe2+ (24 electrons) is not equal to
Bohr's theory is applicable to the following set of species

















