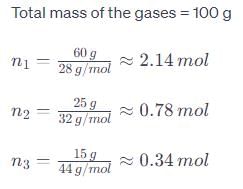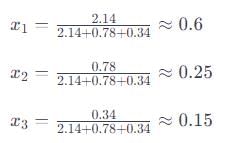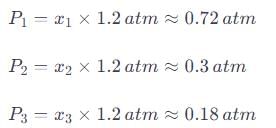Test: Gaseous State - SSC CGL MCQ
10 Questions MCQ Test - Test: Gaseous State
The gaseous state is characterized by the sensitivity of volume change with the change of pressure and temperature due to:
The degree of hotness or coldness of a body is measured by temperature. What is the correct relation between Celsius (C), Kelvin (K), and Fahrenheit (F) scales?
| 1 Crore+ students have signed up on EduRev. Have you? Download the App |
Density is defined as mass per unit volume. What is the correct relation between CGS and SI units for density?
What is the ideal gas equation that relates pressure (P), volume (V), number of moles (n), and temperature (T) for a gas?
What is the correct numerical value of the universal gas constant (R) in liter atmosphere per degree mole?
What is the correct equation to calculate the relative density (vapour density) of a gas with respect to hydrogen?
According to Dalton's Law of Partial Pressure, what is the relationship between total pressure (PT) and partial pressures (P1, P2, ...) of gases in a mixture?
A gaseous mixture contains 60% nitrogen (N2), 25% oxygen (O2), and 15% carbon dioxide (CO2) by mass at a total pressure of 1.2 atm. Calculate the partial pressure of each gas.