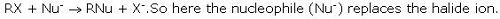Test: Physical and Chemical properties (January 26) - NEET MCQ
10 Questions MCQ Test - Test: Physical and Chemical properties (January 26)
Which one of the following compounds has zero dipole moment?
Which of the following compound does have chiral carbon atom?
| 1 Crore+ students have signed up on EduRev. Have you? Download the App |
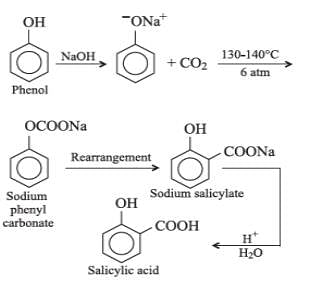
The major product obtanined on interaction of phenol with sodium hydroxide and carbon dioxide is
Which one of the following is an organometallic compound?
Vicinal and geminal dihalides are not distinguished by:
In the nucleophilic substitution reaction in alkyl halides (R-X) , the nucleophile replaces:
Reactivity of alkyl halides towards SN1 nucleophilic substitution reaction is:
Decreasing order of reactivity of alkyl halide is:
The inversion of configuration of optically active alkyl halides occurs in
In dehydrohalogenation reactions the preferred product is that alkene which has the greatest number of alkyl groups attached to the doubly bonded carbon atoms. This rule is known as: