Test: SN1 & SN2 Reactions (January 28) - NEET MCQ
10 Questions MCQ Test - Test: SN1 & SN2 Reactions (January 28)
Which of the following alkyl halides is respectively most and least electrophilic in SN1 reaction?
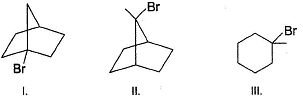
Pick out the compound which reacts fastest in the presence of AgNO3.
| 1 Crore+ students have signed up on EduRev. Have you? Download the App |
Which statement is true about SN2 mechanism?
What is the correct increasing order of reactivity of the followings in SN2 reaction ?
I. CH2 = CHCH2 — Br
II. CH2 = CH— I
III. CH3CH2CH2 — I
IV. CH3OCH2CH2 — I
What is the correct order of reactivity of the followings in hydrolysis reaction at elevated temperature?
The correct statement concerning a SN2 reaction is
One or More than One Options Correct Type
Direction (Q. Nos. 18-22) This section contains 5 multiple choice questions. Each question has four choices (a), (b), (c) and (d), out of which ONE or MORE THAN ONE are correct.
Q.
What is/are true regarding a SN1 reaction?
A SN2 reaction involves back side attack of nucleophile at the α-carbon of substrate because
The correct statement regarding a SN2 reaction is/are
Which of the following can catalyse the following SN1 reaction?

















