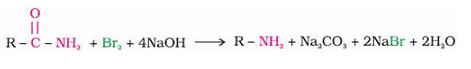NEET Exam > NEET Tests > Test: Preparation of Amines (February 27) - NEET MCQ
Test: Preparation of Amines (February 27) - NEET MCQ
Test Description
10 Questions MCQ Test - Test: Preparation of Amines (February 27)
Test: Preparation of Amines (February 27) for NEET 2024 is part of NEET preparation. The Test: Preparation of Amines (February 27) questions and answers have been prepared
according to the NEET exam syllabus.The Test: Preparation of Amines (February 27) MCQs are made for NEET 2024 Exam.
Find important definitions, questions, notes, meanings, examples, exercises, MCQs and online tests for Test: Preparation of Amines (February 27) below.
Solutions of Test: Preparation of Amines (February 27) questions in English are available as part of our course for NEET & Test: Preparation of Amines (February 27) solutions in
Hindi for NEET course.
Download more important topics, notes, lectures and mock test series for NEET Exam by signing up for free. Attempt Test: Preparation of Amines (February 27) | 10 questions in 20 minutes | Mock test for NEET preparation | Free important questions MCQ to study for NEET Exam | Download free PDF with solutions
Test: Preparation of Amines (February 27) - Question 1
Propanamide on treatment with bromine in an aqueous solution of sodium hydroxide gives:
Detailed Solution for Test: Preparation of Amines (February 27) - Question 1
Test: Preparation of Amines (February 27) - Question 2
We can obtain ethylamine by Hoffmann bromamide reaction. The amide used in this reaction is:
Detailed Solution for Test: Preparation of Amines (February 27) - Question 2
| 1 Crore+ students have signed up on EduRev. Have you? Download the App |
Test: Preparation of Amines (February 27) - Question 3
Nitro compounds are reduced to amines. The catalyst that is preferred is:
Detailed Solution for Test: Preparation of Amines (February 27) - Question 3
Detailed Solution for Test: Preparation of Amines (February 27) - Question 4
Test: Preparation of Amines (February 27) - Question 5
 is a tertiary amine having IUPAC name as:
is a tertiary amine having IUPAC name as:
Test: Preparation of Amines (February 27) - Question 6
Which reaction can be used for the direct conversion of amides into 10 amine ?
Detailed Solution for Test: Preparation of Amines (February 27) - Question 6
Test: Preparation of Amines (February 27) - Question 7
What is the end product in the following sequence of reactions ?
Acetamide  A
A  B
B
Detailed Solution for Test: Preparation of Amines (February 27) - Question 7
Test: Preparation of Amines (February 27) - Question 8
When Primary amide is treated with an aqueous solution of KOH and bromine, it gives a primary amine. The name of the reaction is:
Detailed Solution for Test: Preparation of Amines (February 27) - Question 8
Test: Preparation of Amines (February 27) - Question 9
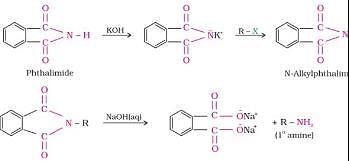
Gabriel phthalimide synthesis is used in the preparation of :
Detailed Solution for Test: Preparation of Amines (February 27) - Question 9
Test: Preparation of Amines (February 27) - Question 10
To convert methyl cyanide to ethylamine we use:
Detailed Solution for Test: Preparation of Amines (February 27) - Question 10
Information about Test: Preparation of Amines (February 27) Page
In this test you can find the Exam questions for Test: Preparation of Amines (February 27) solved & explained in the simplest way possible.
Besides giving Questions and answers for Test: Preparation of Amines (February 27), EduRev gives you an ample number of Online tests for practice
Download as PDF