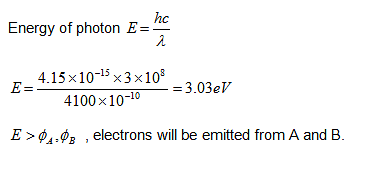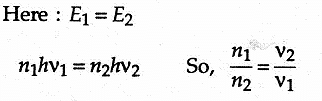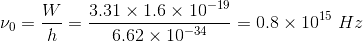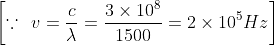Test: Dual Nature of Radiation and Matter - 2 - CUET Humanities MCQ
10 Questions MCQ Test - Test: Dual Nature of Radiation and Matter - 2
Work function of a metal is 5.2 × 10–18. Its threshold wavelength will be
Which one of the following graphs represents the variation of maximum kinetic energy (Ek) of the emitted electrons with frequency (v) in photoelectric effect correctly?
| 1 Crore+ students have signed up on EduRev. Have you? Download the App |
The work functions of metals A, B and C are 1.92 eV, 2.0 eV and 5 eV, respectively. According to Einstein's equation, which metal(s) will emit the photoelectrons for a radiation of wavelength 4100 Å?
There are n1 photons of frequency v1 in a beam of light. In an equally energetic beam, there are n2 photons of frequency v2. The correct relation is
A metal has work function 3.31 eV. It is illuminated by light of wavelength 3 x 10-7m.What is the threshold frequency for photoelectric emission? ( Take h = 6.62 ×10-34 J s)
Threshold wavelength for photoelectric emission, from a metal surface, is 5200 Å. Photoelectrons will be emitted when this surface is illuminated with monochromatic radiation from
A radio transmitter operates on a wavelength of 1500 m at a power of 400 kilowatt. The energy of the radio photon (in joules) is
A metal whose work function is 3.31 eV is illuminated by light of wavelength 3 10-7 m. What is the threshold frequency for photoelectric emission? (Take h = 6.62 x 10-34 Js)
A light having wavelength 300 nm falls on a metal surface. Work function of metal is 2.54 eV. What is the stopping potential?
Relation between wavelength of photon and electron of same energy is