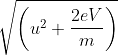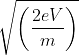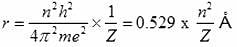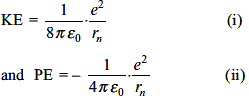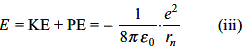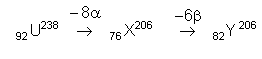Test: Atoms and Nuclei - 1 - CUET Humanities MCQ
10 Questions MCQ Test - Test: Atoms and Nuclei - 1
An electron is accelerated from rest to potential V. The final velocity of the electron is
| 1 Crore+ students have signed up on EduRev. Have you? Download the App |
The total energy of the electron in the first excited state of hydrogen is -3.4 eV. What is the kinetic energy of the electron in this state?
For an electron in the second orbit of hydrogen, the moment of momentum as per Bohr's model is:
A freshly prepared radioactive source of half-life 2 hours emits radiation of intensity which is 64 times the permissible safe level. The minimum time after which it would be possible to work safely with this source is
The binding energies for nuclei 1H1, 2He4, 26Fe56 and 92U235 are 2.22, 28.3, 492 and 1786 MeV, respectively. The most stable nucleus is
What are the respective numbers of α particles and β particles emitted in the following radioactive decay?
In radioactive decay process, the negatively charged emitted β-particles are
If 92U238 emits 8 α-particles and 6 β-particles, then the resulting nucleus is
Fission of nuclei is possible because the binding energy per nucleon in them


 mv2 -
mv2 -  mu2 = qV (∵ W = qV)
mu2 = qV (∵ W = qV) mv2 -
mv2 -  mu2 = eV
mu2 = eV