Test: Thermodynamics Level - 3 - Mechanical Engineering MCQ
25 Questions MCQ Test - Test: Thermodynamics Level - 3
The study of thermodynamics provides answer to the following:
1. whether a process is feasible or not
2. to quantify the energy required for a process
3. rate or speed with which a process occurs
4. extent to which a reaction/process takes place
Which of the above is/are correct?
Consider the following statements:
1. Thermodynamic properties are the macroscopic coordinates significant only for systems existing in states o thermodynamic equilibrium.
2. Engineering thermodynamic studies about storage, transfer and transformation of energy
3. Engineering thermodynamics studies storage, transfer and transformation of energy.
Which of the above is/are correct?
| 1 Crore+ students have signed up on EduRev. Have you? Download the App |
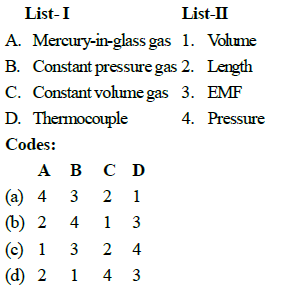
Match the following List- I (Thermometer) with List -II (Thermometric Property):
Convert the following reading of pressure to kPa, assuming that the barometer reads 760 mm of Hg and match the List-I with List-II:
Which of the following aspect is not true regarding microscopic properties of thermodynamic system
Consider the following:
1. Temperature
2. Viscosity3. Specific entropy
4. Thermal conductivity
Which of the above properties of a system is/are intensive?
The value of an extensive property is extensively dependent on
which of the following are intensive properties
1. Kinetic energy 2. Specific enthalpy
3. Pressure 4. Entropy
which of the following statements regarding the concept of continuum are correct?
1. Large number of molecules enable meaningful statistical averaging and assignment of property values
2. Mean free path of the molecules is order of magnitude higher than system dimensions
3. Behaviour of individual molecules is disregarded
4. Mean free path of the molecules approaches the order of magnitude of the system dimensions
Match the following List - I (Type of thermometer) with List -II (Thermometric property) the following :
Experimental data obtained from a constantvolume gas thermometer is shown is the figure below. The value of l in ºC is
The critical point and triple point data for water are
Tc = 374 ºC, Pc = 22.1 MPa
TT = 0.01 ºC PT = 0.6 KPa
Indicate the phase change that will occur in following cases:
(i) Ice at 0.5 Kpa is heated isobarically
(ii) Water vapour at 400ºC is compressed isothermally
A closed system receives 60 kJ heat but its internal energy decreases by 30 kJ. Then the work done by the system is
In a general compression process, 2 kJ of mechanical work is supplied to 4 kg of fluid and 800 J of heat is rejected to the cooling jacket. The change in specific internal energy would be
A paddle wheel used for stirring a liquid contained in a tank supplied 5000 kJ of work and during the stirring operation the tank lost 1500 kJ of heat to the surroundings. If the tank and liquid are considered as a system the change in its internal energy will be
Which one of the following statement bolds good for the equation dQ=dE +dW
300 kJ/s of heat is supplied at a constant fixed temperature of 290ºC to a heat engine. The heat rejection takes place at 8.5ºC. Then match the following.
Match List-I with List-II and select the correct answer using the codes given below.
Match the following :
A. Heat I. State function
B. Internal energy II. Path function
C.Work
D. Entropy
In a cyclic process heat transfer are + 15.7 kJ, - 26.2 kJ, – 4.86 kJ and + 31.5 kJ. What is the net work for this cyclic process?
The value of heat and work transfer for the flow processes of a thermodynamic cycle are given below:
The thermal efficiency and work ratio for the cycle will be respectively
During a thermodynamic process, 84 kJ of heat flows into the system and the work done by the system is 32 kJ. The increase in internal energy of the system is
Given that along the path 1-2-3 a system absorbs 100 kJ as heat and does 60 kJ work while along the pth 1-4-3 it does 20 kJ work (see figure given). The heat absorbed during the cycle 1-4-3 is

















