Test: Thermodynamics Level - 4 - Mechanical Engineering MCQ
25 Questions MCQ Test - Test: Thermodynamics Level - 4
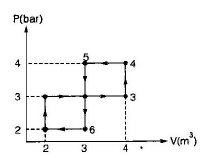
The network output for the cycle 1-2-3-4-5-6-1 shown in figure is
Consider the following statement about steady flow process:
1. The rate of flow of mass energy across the control surface are constant.
2. Thermodynamic properties vary along space as well as time coordinates.
3. Any thermodynamic property will have a fixed value at a particular location and will not alter with time.
Which of the above are correct ?
| 1 Crore+ students have signed up on EduRev. Have you? Download the App |
The term m Dh in a control volume equation Q – W = mDh
Select the Kelvin-Plank statement of the second law:
According to the Clausiu’s statement of the second law:
1. heat flows from cold surface to hot surface, unaided.
2. heat flows from hot surface to cold surface, unaided.
3. heat can flow from cold surface to hot surface with the aid of external work.
Which of the above statement is /are correct ?
The more effective way of increasing the efficiency of a carnot engine is to
Consider the following statements:
1. It is the second law of thermodynamics which provides criterion as to the probability of various processes.
2. Heat and work are completely interchangeable forms of energy.
3. Work is high grade energy.
4. The complete conversion of low grade energy into high grade energy in a cycle is impossible
Which of the above statements are correct ?
Perpetual motion machine of second kind violates the
The efficiency of the carnot cycle may be increased by
An engine operates between temperature limits of 900 K and T2 and another between T2 and 400 K for both to be equally efficient T2 will be equal to
For the same above engine, if work output is to be equal than T2 will be equal to
Three engines A, B and C operating on carnot cycle respectively use air, steam and Helium as the working fluid. If all the engine operates within the same temperature limit, then which engine will have highest efficiency
If a heat engine gives an output of 3 kW when the input is 10000 J/sec then thermal efficiency of the engine will be
The pressure’of a gas in terms of its mean kinetic energy per unit volume E is equal to
Match List - I ( Laws of thermodynamics) with List -II (Defines) the following:
The slope of constant pressure line on a T-S diagram is given by
In statistical thermodynamics, entropy is defined as
1. Measure of irreversibility of a system
2. A universal property
3. Degree of randomness
4. Thermodynamic probability of disorderness
Which of the above is/are correct ?
Consider the following statements: In an irreversible process
1. entropy always increases
2. the sum of the entropy of all bodies taking part in a process always increases
3. Once created, entropy can not be destroyed
Which among the above is /are correct ?
One kg of water at room temperature is brought into contact with a high temperature thermal reservoir. The entropy change of the universe is
For a thermodynamics cycle to be irreversible, it is necessary that
The entropy change for any closed system which undergoes an irreversible adiabatic process
Consider two subsystem 1 and 2 containing same fluid and having same mass m; but at Temperature T1 and T2 (T1 > T2) enclosed in an adiabatic enclosure separate by a partition. If the partition is removed and the fluids are allowed to mix. The entropy change of process is

















