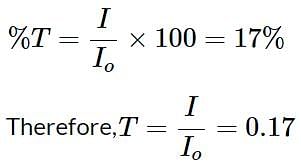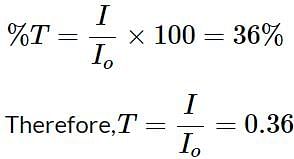Test: Physical Spectroscopy - 1 - Chemistry MCQ
10 Questions MCQ Test - Test: Physical Spectroscopy - 1
What is the absorbance of an IR peak with a 17% transmittance?
What is the absorbance of an IR peak with a 36% transmittance?
| 1 Crore+ students have signed up on EduRev. Have you? Download the App |
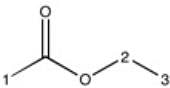
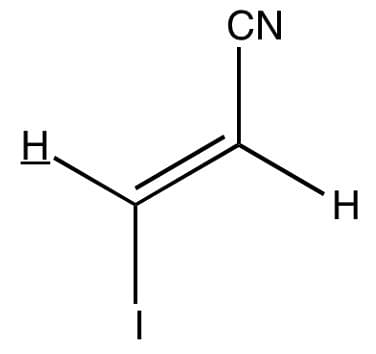
Which of the following most likely represents the H-NMR spectrum of the molecule shown below?
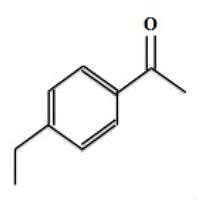

Which of the following observations would most likely be seen when performing an H-NMR on 1-ethyl ethanoate (below)?
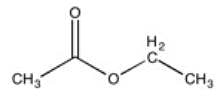
All of the following molecules would exhibit two distinct singlets in a 1H-NMR spectrum except __________.
Observe the underlined/bold hydrogen. In HNMR, how many spectral lines will that bolded hydrogen be split into?
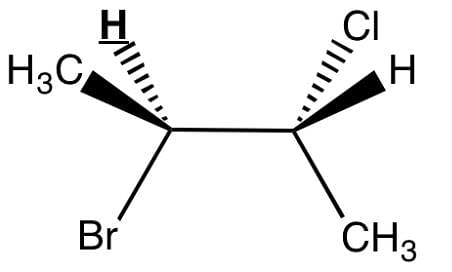
Observe the underlined/bold hydrogen. In HNMR, how many spectral lines will that bolded hydrogen be split into?
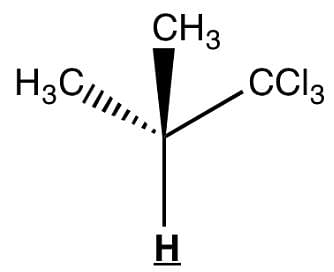
Observe the underlined/bold hydrogen. In HNMR, how many spectral lines will that bolded hydrogen be split into?
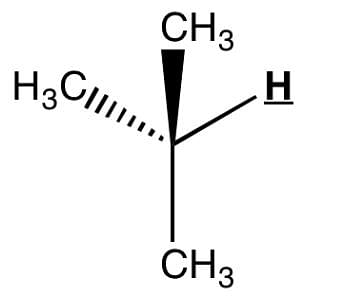
Observe the underlined/bold hydrogen. In HNMR, how many spectral lines will that bolded hydrogen be split into?
Which of the following spectroscopic techniques provides the most information about an organic molecule's framework/structure?