Test: Mole Concept & Molar Mass (April 7) - JEE MCQ
10 Questions MCQ Test - Test: Mole Concept & Molar Mass (April 7)
For the reaction 2x + 3y + 4z → 5w
Initially if 1 mole of x, 3 mole of y and 4 mole of z is taken. If 1.25 mole of w is obtained then % yield of this reaction is
Molarity of NaOH in a solution prepared by dissolving 4 g of NaOH in enough water to form 250 ml of solution is:
| 1 Crore+ students have signed up on EduRev. Have you? Download the App |
125 ml of 8% w/w NaOH solution (sp. gravity 1) is added to 125 ml of 10% w/v HCl solution. The nature of resultant solution would be ____
Ratio of masses of H2SO4 and Al2 (SO4)3 is grams each containing 32 grams of S is _____
18 g of glucose (C6H12O6) is present in 1000 g of an aqueous solution of glucose. The molality of this solution is:
For the reaction 2A + 3B + 5C → 3D
Initially if 2 mole of A, 4 mole of B and 6 mole of C is taken, With 25% yield, moles of D which can be produced are _____________.
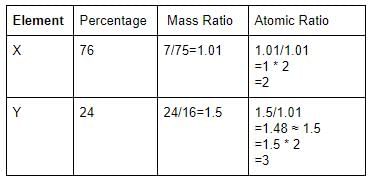
Two elements X (atomic mass = 75) and Y (atomic mass = 16) combine to give a compound having 75.8% of X. The formula of the compound is:
Equal volumes of 10% (v/v) of HCl is mixed with 10% (v/v) NaOH solution. If density of pure NaOH is 1.5 times that of pure HCl then the resultant solution be :
Similar to the % labelling of oleum, a mixture of H3PO4 and P4O10 is labelled as (100 + x) % where x is the maximum mass of water which can react with P4O10 present in 100 gm mixture of H3PO4 and P4O10. If such a mixture is labelled as 127% Mass of P4O10 is 100 gm of mixture, is
C6H5OH(g) + O2(g) → CO2(g) + H2O(l)
The magnitude of volume change if 30 ml of C6H5OH (g) is burnt with an excess amount of oxygen, is