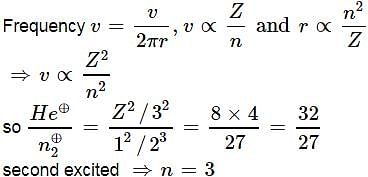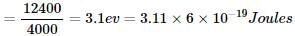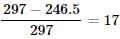Test: Structure of the Atom - 2 - NEET MCQ
10 Questions MCQ Test - Test: Structure of the Atom - 2
Most penetrating radiation of radioactive element is:
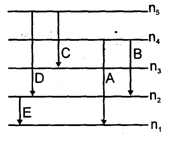
For a hypothetical H like atom which follows Bohr's model, some spectral lines were observed as shown. If it is known that line 'E' belongs to the visible region, then the lines possibly belonging to ultra violet region will be (n1 is not necessarily ground state)
[Assume for this atom, no spectral series shows overlaps with other series in the emmission spectrum]
| 1 Crore+ students have signed up on EduRev. Have you? Download the App |
The number of photons emitted in 10 hours by a 60W sodium lamp (λ, of photon = 6000 Å)
[Take he = 12400 EvÅ, h = Planck's constant, c = speed of light]
Radius of 3rd orbit of Li2+ ion is ‘x’ cm then de-broglie wavelength of electrons in the 1st orbit is
Ratio of frequency of revolution of electron in the second excited state of He⊕and second state of hydrogen is
In any subshell, the maximum number of electrons having same value of spin quantum number is:
A proton accelerated from rest through a potential difference of 'V' volts has a wavelength λ associated with it. An alpha particle in order to have the same wavelength must be accelerated from rest through a potential difference of
Consider the ground state of Cr atom (Z = 24). The numbers of electrons with the azimuthal quantum numbers, = 1 and 2 are, respectively:
Number of electrons having l + m value equal to zero in 26Fe may be
A photon of 4000 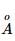 is used to break the iodine molecule, then the % of energy converted to the K.E. of iodine atoms if bond dissociation energy of I2 molecule is 246.5 kJ/mol is:
is used to break the iodine molecule, then the % of energy converted to the K.E. of iodine atoms if bond dissociation energy of I2 molecule is 246.5 kJ/mol is: