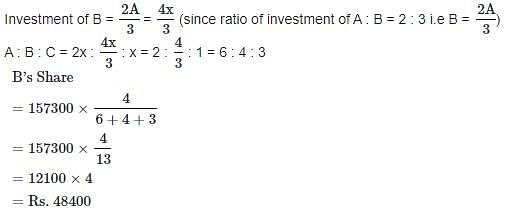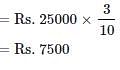DSSSB PGT Chemistry Mock Test - 1 - DSSSB TGT/PGT/PRT MCQ
30 Questions MCQ Test - DSSSB PGT Chemistry Mock Test - 1
Direction: Read the following passages carefully and answer the question that follows.
Seven-year -old Jim came home from the park without his new bicycle. "An old man and a little boy borrowed it. " he explained. "They are going to bring it back at four O'clock,". His parents were upset that he had given his expensive new bicycle, but were secretly proud of his kindness and faith. Came four O'clock, no bicycle. The parents were anxious. But at 4 :30, the door bell rang. and there stood a happy man and a boy , with the bicycle and a box of chocolates. Jim suddenly disappeared into his bedroom, and then came running out. ' All right , ' he said, after examining the bicycle. "You can have your watch back ! " .
Q. When jim came home without his bicycle, his parents?
Seven-year -old Jim came home from the park without his new bicycle. "An old man and a little boy borrowed it. " he explained. "They are going to bring it back at four O'clock,". His parents were upset that he had given his expensive new bicycle, but were secretly proud of his kindness and faith. Came four O'clock, no bicycle. The parents were anxious. But at 4 :30, the door bell rang. and there stood a happy man and a boy , with the bicycle and a box of chocolates. Jim suddenly disappeared into his bedroom, and then came running out. ' All right , ' he said, after examining the bicycle. "You can have your watch back ! " .
Direction: Read the following passages carefully and answer the question that follows.
We know that gold and silver are elements. An element consists of atoms of only one kind, unlike water, which consists of both hydrogen and oxygen. Another element is radium. Radium is especially interesting, because it produces heat, When soil is carried by rivers down to the sea and falls to the bottom, it often carries radium in it. As more and more soil is washed down into the ocean. more and more radium is carried in it. There, as elsewhere, it constantly produces heat.
Q. Some heat on the ocean floor comes from?
We know that gold and silver are elements. An element consists of atoms of only one kind, unlike water, which consists of both hydrogen and oxygen. Another element is radium. Radium is especially interesting, because it produces heat, When soil is carried by rivers down to the sea and falls to the bottom, it often carries radium in it. As more and more soil is washed down into the ocean. more and more radium is carried in it. There, as elsewhere, it constantly produces heat.
| 1 Crore+ students have signed up on EduRev. Have you? Download the App |
Direction: Read the following passage carefully and answer the questions. Your answer to these questions should be based on passage only.
In a country where more than 400 million people live without electricity, it isn't political rhetoric and policies filtered through many layers of bureaucracy that will benefit people. An innovative approach to identifying and addressing the basic lack of infrastructure works best. This is best exemplified by the initiative to convert rice husks into electricity in Bihar. Pioneered by a company called Husk Power Systems, established in 2007, by Bihar native Gyanesh Pandey along with three partners- it's a peculiarly Indian initiative, making a virtue out of the necessity of low-cost solutions to large scale problems. In essence, Pandey and his partners have devised an electric distribution system powered by the waste product of rice husks, generated when rice is milled- something in abundant supply in Bihar where, it is estimated, 1.8 billion kg of rice husk are produced annually
Q. What is the best initiative taken?
In a country where more than 400 million people live without electricity, it isn't political rhetoric and policies filtered through many layers of bureaucracy that will benefit people. An innovative approach to identifying and addressing the basic lack of infrastructure works best. This is best exemplified by the initiative to convert rice husks into electricity in Bihar. Pioneered by a company called Husk Power Systems, established in 2007, by Bihar native Gyanesh Pandey along with three partners- it's a peculiarly Indian initiative, making a virtue out of the necessity of low-cost solutions to large scale problems. In essence, Pandey and his partners have devised an electric distribution system powered by the waste product of rice husks, generated when rice is milled- something in abundant supply in Bihar where, it is estimated, 1.8 billion kg of rice husk are produced annually
Direction: Read the following passage and answer the questions based on it.
Honest self-criticism plays a vital role to purify our souls and to light the path of blissful success. The Holy Quran says: Truly he has succeeded who purifies it. And Truly, he has failed who defiles it. In fact, self criticism seems like a fairly straight-forward concept. It means acknowledging that we have committed a sin, whether against ourselves or other, be it Our Creator or anyone or anything in creation. For most of us, such a confession is an incredibly tough thing to do. Pride prevents us from owning our faults, especially before people when it is necessary. It behoves us to recall that being honest with ourselves is actually a way to enjoy life, rather than make it tougher. It is a fact that the best way to prevent ourselves from committing haram acts is to really investigate whether or not such activities are permissible in Islam and to refrain from doing them if they are not permissible
Q. What does self-criticism mean in this passage ?
Sheldon had to cover a distance of 60 km. However, he started 6 minutes later than his scheduled time and raced at a speed 1 km/h higher than his originally planned speed and reached the finish at the time he would reach it if he began to race strictly at the appointed time and raced with the assumed speed. Find the speed at which he travelled during the journey described.
In a business, A and C invested amounts in the ratio 2 : 1 , whereas the ratio between amounts invested by A and B was 3 : 2 . If Rs 157300 was their profit, how much amount did B receive?
A and B started a business in partnership investing Rs. 20,000 and Rs. 15,000 respectively. After six months, C joined them with Rs. 20,000. What will be B's share in total profit of Rs. 25,000 earned at the end of 2 years from the starting of the business?
A tablet is sold for ₹ 6612.5 at a profit of 15%. What would have been the actual profit or loss on it, if it had been sold for ₹ 5380?
Three metal cubes having volumes 125 cubic cm, 64 cubic cm and 27 cubic cm are melted to form a new cube. Find the value of edge of the new cube.
If p = – 7 and q = 12 and x2+px+q=0, then the value of ‘x’ is
वे प्रत्यय जो क्रिया में जुड़े होते हैं उन्हें कहते हैं-
Direction (Q. Nos. 1-18) This section contains 18 multiple choice questions. Each question has four
choices (a), (b), (c) and (d), out of which ONLY ONE option is correct.
Q. Oxidation can be defined as the terms
I. gain of electron and hydrogen
II. gain of oxygen and loss of electron
III. increase in oxidation number
IV. decrease in oxidation number
Select the correct terms
Following cell has EMF 0.7995 V.
Pt | H2 (1 atm) | HNO3 (1M) || AgNO3 (1M) | Ag
If we add enough KCl to the Ag cell so that the final Cl- is 1M. Now the measured emf of the cell is 0.222 V.
The Ksp of AgCl would be :
Only One Option Correct Type
This section contains 10 multiple choice questions. Each question has four choices (a), (b), (c) and (d), out of which ONLY ONE is correct
Which of the following concentration factors is affected by change in temperature?
6.02 x1020 molecules of urea are present in 100 mL of its solution. The concentration of urea solution is (N0 = 6.02 x 1023 mol-1)
[AIEEE 2004]
The decreasing order of the repulsive interactions between various electron pairs is:
Direction (Q. Nos. 1-16) This section contains 16 multiple choice questions. Each question has four choices (a), (b), (c) and (d), out of which ONLY ONE option is correct.
Q.
The shape [CIF4]- and [ClF2]- ions is respectively
Direction (Q. Nos. 1-12) This section contains 12 multiple choice questions. Each question has four choices (a), (b), (c) and (d), out of which ONLY ONE option is correct.
Q. Which of the following compounds are soluble in H2O ?
Which among the following compound, nitrogen shows the oxidation state of +5?
Direction (Q. Nos. 1-7) This section contains 7 multiple choice questions. Each question has four choices (a), (b), (c) and (d), out of which ONLY ONE is correct.
Q.
The hybridisation of N in solid state for N2O5 is
Direction (Q. Nos. 1-10) This section contains 10 multiple choice questions. Each question has four choices (a), (b), (c) and (d), out of which ONLY ONE is correct.
Q.
Which is incorrect statement?
Direction (Q. Nos. 1-10) This section contains 10 multiple choice questions. Each question has four choices (a), (b), (c) and (d), out of which ONLY ONE is correct.
Q.
Which of the following property decreases down the group in the halogens?
Which of the following represents chelating ligand?
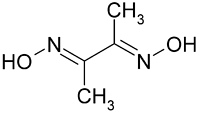
Correct IUPAC name of the following compound is :
Direction (Q. Nos. 1-18) This section contains 18 multiple choice questions. Each question has four
choices (a), (b), (c) and (d), out of which ONLY ONE option is correct.
Q.
The correct statement regarding a chiral compound is
Direction (Q, Nos. 1 - 5) This section contains 5 multiple choice questions. Each question has four choices (a), (b), (c) and (d), out of which ONLY ONE option is correct.
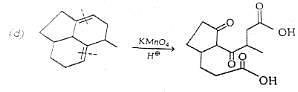
Q. Which molecule will give the following dicarboxylic acid upon treatment with acidic solution of KMnO4?
The uncertainty found from the uncertainty principle (Δx.Δp = h/4 π) is