EmSAT Chemistry Mock Test- 5 - EmSAT Achieve MCQ
30 Questions MCQ Test - EmSAT Chemistry Mock Test- 5
| 1 Crore+ students have signed up on EduRev. Have you? Download the App |
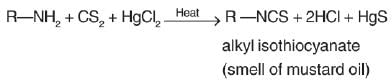
Primary amine reacts with carbon di sulphide in presence of excess of mercuric chloride and produce isothiocyanate. This reaction is known as
Which of the following reacts with chloroform and base to form phenyl isocyanide.
hydrocarbon is
0.7 g of is dissolved in 10 mL of water, 20mL of which required 19.8 mL of 0.1 N HCl. The value of x is
The energy of an electron in the first Bohr orbit of H-atom is –13.6 eV. The possible energy values(s)
of the excited state(s) for electrons in Bohr orbits of hydrogen is (are)
If n and l are respectively the principal and azimuthal quantum numbers, then the expression for calculating total number of electrons in any energy level is
Traid-II
Arrange the elements with the following electronic configurations in increasing order of electron affinity