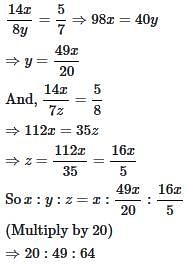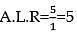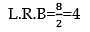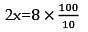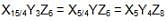DSSSB PGT Chemistry Mock Test - 7 - DSSSB TGT/PGT/PRT MCQ
30 Questions MCQ Test - DSSSB PGT Chemistry Mock Test - 7
Directions to Solve
In each of the following questions there are three statements. Which are followed by three or four conclusions. Choose the conclusions which logically follow from the given statements.
Question -
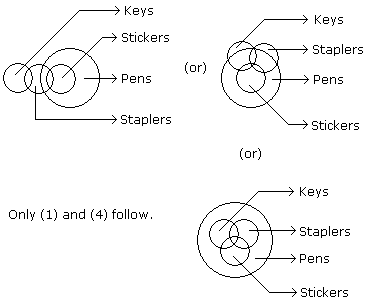
Statements: Some keys are staplers. Some staplers are stickers. All the stickers are pens.
Conclusions:
- Some pens are staplers.
- Some stickers are keys.
- No sticker is key.
- Some staplers are keys.
Pointing to a photograph of a boy Suresh said, "He is the son of the only son of my mother." How is Suresh related to that boy?
| 1 Crore+ students have signed up on EduRev. Have you? Download the App |
If LMNO is written as GHIJ, how will STUV be written?
The graphical solution of -1 < x < 2 on number line is
Romantically called, “the war of the Goldsmith’s daughter”, took place between
The Dutch and the English entered the East as friend against the common enemy, the Portuguese. However, their commercial rivalry led to the massacre of the Englishman by the Dutch at
The Dutch and the English entered the East as friend against the common enemy, the Portuguese. However, their commercial rivalry led to the massacre of the Englishman by the Dutch at
Directions to Solve:
Choose the correct alternative that will continue the same pattern and replace the question mark in the given series.
Question. 120, 99, 80, 63, 48, ?
Three partners shared the profit in a business in the ratio 5 : 7 : 8. They had partnered for 14 months, 8 months and 7 months respectively. What was the ratio of their investments?
A sum of money invested at simple interest triples itself in 8 years at simple interest. Find in how many years will it become 8 times itself at the same rate?
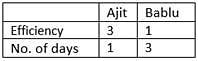
Ajit is 3 times as efficient as Bablu, then the ratio of number of days required by each to work alone, completely?
निर्देश: निम्नलिखित शब्द के लिए उसके नीचे दिए गए विकल्पों में से सही पर्यायवाची शब्द चुनकर उत्तर दीजिए।
कैवल्य
मेघ आए बड़े बन ठन के सँवर के 'पाहुन ज्यों आए हों गाँव में शहर के' पंक्तियों में कौन साअलंकार है ?
For a reaction A + 2B → C, the amount of C formed by starting the reaction with 5 moles of A and 8 moles of B is
Acrystal is made of particles A and B. A forms FCC packing and B occupies all the octahedral voids. If all the particles along the plane as shown in figure are removed, then, the formula of the crystal would be:
A crystal is made of particle X, Y & Z. X forms FCC packing, Y occupies all octahedral voids of X and Z occupies all tetrahedral voids of X, if all the particles along one body diagonal are removed then the formula of the crystal would be –
In a hypothetical solid C atoms are found to form cubical close packed lattice. A atoms occupy all tetrahedral vioids & B atoms occupy all octahedral voids. A and B atoms are of appropriate size, so that there is no distortion in CCP lattice of C atoms. Now if a plane as shown in the following figure is cut.Then the cross section of this plane will look like.
Energy of H–atom in the ground state is -13.6 eV , Hence energy in the second excited state is–
Direction (Q. Nos. 14 and 15) Choice the correct combination of elements and column I and coloumn II are given as option (a), (b), (c) and (d), out of which ONE option is correct.
Q.
Match the equation in Column I with the name type in Column II.
Which of the following statements is/are correct?
1 M NaCl and 1 M HCl are present in an aqueous solution. The solution is
Substances that alter the rate of a chemical reaction without being used up in a chemical reaction is known as
What is aggregation of colloidal particles into insoluble precipitate by addition of some suitable electrolyte called?
Which among the following is an instantaneous reaction?