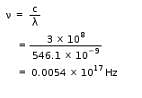Test: Hydrogen Spectrum (April 22) - JEE MCQ
10 Questions MCQ Test - Test: Hydrogen Spectrum (April 22)
Calculate the wavelength of light that corresponds to the radiation that is given off during the transition of an electron from the n = 5 to n = 2 state of the hydrogen atom.
| 1 Crore+ students have signed up on EduRev. Have you? Download the App |
The total energy of an electron in the nth orbit of a hydrogen atom is given by the formula En = -13.6 eV/n2. What does the negative energy for an electron indicate?
In the absorption spectrum, the wavelengths which are absorbed, are missing and they appear as:
By use of a suitable filter, the green mercury emission line can be isolated. This line has a wavelength of 546.1 nm. What is the frequency? [1 Hz = 1 s-1]
An electron falls from one energy level to another; it releases a certain amount of light with a frequency of 5.100 x 1014 Hz. What energy is associated with this electron?
The energy associated with the transition of an electron from the n=1 state to the n=3 state of H atoms is:
How much energy is needed to ionize a hydrogen atom if electron is present in n=1 orbit?
Zeeman effect is the splitting of spectral line in presence of: