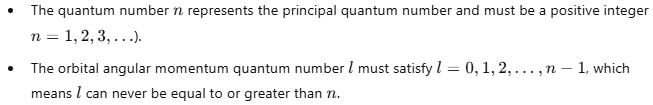Test: Quantum Numbers (April 24) - JEE MCQ
10 Questions MCQ Test - Test: Quantum Numbers (April 24)
For a multi-electron atom, set of quantum numbers is given as
2,0,0,1/2 ; 2,0,0,-1/2
Q. Thus, the next higher allowed set of n and / quantum numbers for this atom in its ground state is
The orbital angular momentum for a d-orbital electron is given by
| 1 Crore+ students have signed up on EduRev. Have you? Download the App |
A hydrogen like species in fourth orbit has radius 1.5 times that of Bohr's orbit. In neutral state, its valence electron is in
The correct set of four quantum numbers for the valence electron of rubidium atom (Z = 37) is
[JEE Main 2013]
Last filling electron in lanthanides find place in 4f-orbital. Which of the following sets of quantum number is correct for an electron in 4f orbital?
In the following pair, each has two orbitals I and II. Select the ones in which II experiences larger effective nuclear charge than l
Which of the following statements is/are correct?
Which of the following sets of quantum numbers (n, l, m, s) is not possible for an electron in an atom?
The maximum number of electrons that can have principal quantum number, n = 3 and spin quantum number,