Revisal Problems (Past 13 Years) JEE Advanced (Chemical Bonding) - JEE MCQ
30 Questions MCQ Test - Revisal Problems (Past 13 Years) JEE Advanced (Chemical Bonding)
Only One Option Correct Type
Direction (Q, Nos. 1-5) This section contains 5 multiple choice questions. Each question has four choices (a), (b), (c), and (d), out of which ONLY ONE option is correct.
Q.
Select correct matching about [Ni(CN)4]2- , Ni(CO)4, [NI(H2O)4)]2+

Energy gap between the highest filled bands and the lowest empty bands in elements are given :
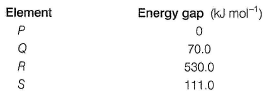
Thus,
| 1 Crore+ students have signed up on EduRev. Have you? Download the App |
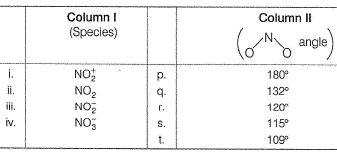
Match Column I with Column II and select the correct answer from the codes given below the table.

Hybridisation of the underlined atoms is/are affected in the following transformations :
In BrF3 molecule, the lone pairs occupy equatorial positions to minimise
Statement Type
Direction (Q. Nos. 6 - 8) This section is based on Statement I and Statement II. Select the correct answer from the codes given below.
Q.
Statement I : Two different bond lengths are observed in PF5 molecule but only one bond length is observed in SF6 molecule.
Statement II : One lone pair exists in PF5
Statement I : The ion CIF2- is linear but the ion CIF2+ is bent.
Statement II : Cl-atom is sp2-hybridised in each.
Statement I : Carbon and silicon are in group 14 in but ( Si=Si) bond is not formed but (C=C) bond is formed.
Statement II : Larger size of Si results in poor sideway overlap of p-orbitals to form π-bond.
One or More than One Options Correct Type
Direction (Q. Nos. 9-21) This section contains 13 multiple choice questions. Each question has four choices (a), (b), (c), and (d), out of which ONE or MORE THAN ONE are correct.
Q.
Select correct statement(s)
Select the correct variation of solubility of different salts in water.
Which of the following molecules is expected to exhibit diamagnetic behaviour?
Which is/are correct variation (s) for
Which is/are not correct variation
The hybridisation scheme for the central atom includes ad-orbital contribution in
Select the correct statement(s).
One or more species out of the following uses sp2-hybrid orbitals in bonding is/are
Consider the following species :
Sets of species with same type of hybridisation on underlined atom is/are
In which of the following cases, driving force is to complete the octet of B-atom permanently
Which of the follow ing m olecules has the m axim um num ber of A—X bonds of identical bond length when A is the central atom and X is the surrounding atom?
Select the correct statement(s) about PCI5,
Which of the following structures assume linear structure?
Comprehension Type
Direction (Q. Nos. 22-25) This section contains 2 paragraphs, each describing theory, experiments, data, etc. four questions related to the paragraphs have been given. Each question has only one correct answer among the four given options (a), (b), (c) and (d).
Passage l
Homolytic and heterolytic bond cleavage of organic compounds may result in short lived intermediates.
Q.
Hybridisation of C-atom in the species formed is of the type
Passage l
Homolytic and heterolytic bond cleavage of organic compounds may result in short lived intermediates.
Q.
Bond angle in the increasing order is
Passage II
Consider the following compounds of nitrogen and answer the given questions :
Q.
Hybridisation of nitrogen atom can be
Passage II
Consider the following compounds of nitrogen and answer the given questions :
Q.
Structures can be
I. Trigonal planar
II. Trigonal pyramidal
III. Square planar
Select the correct structures
Matching List Type
Direction (Q. Nos. 26 and 27) Choices for the correct combination o f elements from Column I and Column II are given as options.
Q.
Match the reactions in Column I with the nature of reactions/type of the products in Column II.
[IITJEE2007]
Match each of the diatomic molecules in Column I with the property/properties in Column II.
[IITJEE 2009]
One Integer Value Correct Type
Direction (Q. Nos. 28-30) This section contains 3 questions. When worked out will result in an integer from 0 to 9 (both inclusive).
Q.
At 300 K and1.00 atm, density of gaseous HF is 3.17 gL-1. How many HF molecules are associated by H-bonding?
AX4 forms square planar type structure. Distance between A and X-atoms is 4 units. What is distance between two X-atoms in trans-position?
A total of n x1020 energy levels are present in 3s conduction band of single crystal of sodium weighing 26.8 mg. What is the value of n?

















