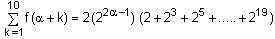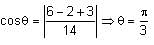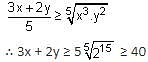COMEDK Mock Test - 1 - JEE MCQ
30 Questions MCQ Test - COMEDK Mock Test - 1
When the current in the portion of the circuit, shown in the figure, is 2 A and is increasing at the rate of 1 A/s, then the measured potential difference Vab is 8 V. However, when the current is 2 A and is decreasing at the rate of 1 A/s, the measured potential difference Vab is 4V. The values of R and L are


Two beams of red and violet colors are made to pass separately through a prism (angle of the prism is 60°). In the position of minimum deviation, the angle of refraction will be:
| 1 Crore+ students have signed up on EduRev. Have you? Download the App |
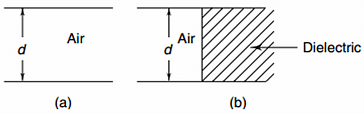
A parallel plate air-filled capacitor has a capacitance of 2 μF . When it is half-filled with a dielectric of dielectric constant k = 3, its capacitance becomes

To raise the temperature of a certain mass of gas by 50°C at a constant pressure, 160 calories of heat is required. When the same mass of gas is cooled by 100°C at constant volume, 240 calories of heat is released. How many degrees of freedom does each molecule of this gas have (assume gas to be ideal)?
The magnetic field associated with a light wave is given, at the origin, by B = B0 [sin(3.14 × 107)ct + sin(6.28 × 107)ct]. If this light falls on a silver plate having a work function of 4.7 eV, what will be the maximum kinetic energy of the photo electrons ?
(c = 3 × 108 ms-1, h = 6.6 × 10-34 J-s)
Value of gas constant, R for one mole of a gas is independent of the
A long solenoid carrying a current produces a magnetic field B along its axis. If the current is doubled and the number of turns per cm is halved, the new value of magnetic field will be equal to
When a conductor is placed in an electric field; its free charge carriers adjust itself in order to oppose the electric field. This happen until
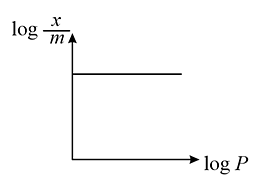
The above graph for Freundlich adsorption isotherm will be true when:
The following equilibria are given:
a. N2 + 3H2 2NH3; K1
b. N2 + O2 2NO; K2
c. H2 + O2
H2O; K3
The equilibrium constant for the reaction,
2NH3 + O2
2NO + 3H2O in terms of K1, K2 and K3 will be
In the Newman projection for 2, 2-dimethylbutane, X and Y, respectively, will be

A. H and H
B. H and C2H5
C. C2H5 and H
D. CH3 and CH3
Considering H2O as a weak field ligand, the number of unpaired electrons in [Mn(H2O)6]2+ will be
(Atomic number of Mn = 25)
Acid hydrolysis of which of the following compounds yields two different organic compounds?
One mole of an ideal gas for which Cv = 3/2 R is heated reversibly at a constant pressure of 1 atm from 25°C to 100°C. Here, H (the value in Calories to be provided correct upto two decimal places) is
Isopropylbenzene, on air oxidation in the presence of dilute acid, gives
The standard enthalpy of neutralisation of HCl by NaOH is -57.62 kJ mol-1, which is the enthalpy of formation of water.
The standard enthalpy of neutralisation of HCN by NaOH is
Let f : N → R be a function such that f(x + y) = 2f(x) f(y) for natural numbers x and y. If f(1) = 2, then the value of α for which  holds, is
holds, is
Let A and B be two independent events such that P(A) = 1/3 and P(B) = 1/6. Then which of the following is TRUE?
Consider the following two propositions:
P1 : ~(p → ~q)
P2 : (p ∧ ~q) ∧ ((~p) ∨ q)
If the proposition p → ((~p) ∨ q) is evaluated as FALSE, then:
Let P be the plane passing through the intersection of the planes 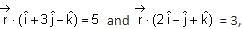 and the point (2, 1, -2). Let the position vectors of the points X and Y be and , respectively. Then the points
and the point (2, 1, -2). Let the position vectors of the points X and Y be and , respectively. Then the points
Let the points on the plane P be equidistant from the points (-4, 2, 1) and (2, -2, 3). Then the acute angle between the plane P and the plane 2x + y + 3z = 1 is:
If the real part of the complex number (1 - cosθ + 2i sinθ)-1 is 1/5 for θ ∈ (0, π), then the value of the integral  dx is equal to:
dx is equal to:
Let x, y > 0. If x3y2 = 215, then the least value of 3x + 2y is
Let λ * be the largest value of λ for which the function fλ(x) = 4λx3 - 36λx2 + 36x + 48 = is increasing for all x ∈ R. Then, fλ * (1) + fλ* (-1) is equal to:



 for both the colour.
for both the colour.


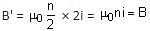
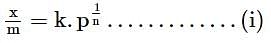









 ion, sulfur atoms are located as center atoms and oxygen atoms are located around sulfur atoms. There are two double bonds around a sulfur atom in the lewis structure. Oxidation number of sulfur is six in
ion, sulfur atoms are located as center atoms and oxygen atoms are located around sulfur atoms. There are two double bonds around a sulfur atom in the lewis structure. Oxidation number of sulfur is six in  ion.
ion. has no s−s linkage :
has no s−s linkage :