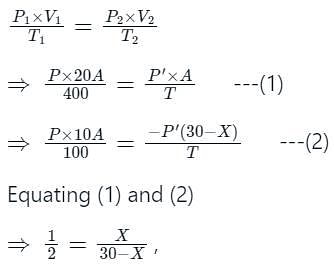Test: Thermal Equilibrium - EmSAT Achieve MCQ
10 Questions MCQ Test - Test: Thermal Equilibrium
A system is in thermal equilibrium with its environment if-
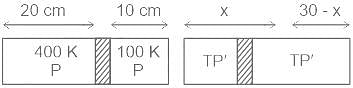
A cylindrical tube of a length of 30 cm is partitioned by a tight-fitting separator. The separator is very weakly conducting and can freely slide along the tube. Ideal gases are filled in the two parts of the vessel. In the beginning, the temperatures in parts A and B are 400 K and 100 K respectively. The separator slides to a momentary equilibrium position shown in the figure. Find the final equilibrium position of the separator, reached after a long time.

| 1 Crore+ students have signed up on EduRev. Have you? Download the App |
A solid X is in thermal equilibrium with a solid Y. Solid Y has the same temperature as solid Z. If the three solids have different masses and compositions, which one of the following statements is correct?
Two bodies in contact are said to be in thermal equilibrium when
If __________ between the system and the surroundings, the process is adiabatic.
Any process in which the system returns to its initial state after undergoing a series of changes is known as ___________
An ideal gas heat engine is operating between 227°C and 127°C. It absorbs 104 J of heat at the higher temperature. The amount of heat converted into work is?