Bihar PGT Chemistry Mock Test - 1 - Bihar PGT/TGT/PRT MCQ
30 Questions MCQ Test - Bihar PGT Chemistry Mock Test - 1
In the following question, out of the four alternatives, select the alternative which is the best substitute for the phrase.In the following question, out of the four alternatives, select the alternative which is the best substitute for the phrase.
Q. A person who does not believe in the existence of God.
Improve the bracketed part of the sentence with the parts given below.
Q. He (turned over a new leaf) after serving his remaining prison sentence
| 1 Crore+ students have signed up on EduRev. Have you? Download the App |
Directions: In the following question, a sentence has been given in Active/Passive voice. Out of four alternatives suggested, select the one which best expresses the same sentence in Passive/Active voice.
Q. Alice posted the letter.
Arrange the following words in the logical and meaningful order.
1. Designing
2. Manufacturing
3. Production
4. Planning
5. Implementation
Excessive consumption of alcoholic drinks causes damage to the
Only One Option Correct Type
This section contains 16 multiple choice questions. Each question has four choices (a), (b), (c) and (d), out of which ONLY ONE is correct
Q.
During the electrolysis of aqueous Zn(NO3)2 solution
Only One Option Correct Type
Direction (Q. Nos. 1- 13) This section contains 13 multiple choice questions. Each question has four choices (a), (b), (c) and (d), out of which ONLY ONE option is correct.
Consider the following reaction,
Q.
the major product in the above reaction is
100 mL of aqueous solution of 0.01 M CaCI2 is evaporated to dryness when 0.15 g of residue is obtained. Thus, impurity present is
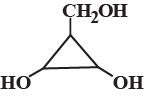
The number of optically active optical isomers of the compound is:
Direction (Q. Nos. 1-7) This section contains 7 multiple choice questions. Each question has four choices (a), (b), (c) and (d), out of which ONLY ONE is correct.
Q.
The hybridisation of N in solid state for N2O5 is
For the reaction equilibrium :
N2O4 (g) 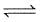 2NO2(g) ; the concentration of N2O4 and NO2 at equilibrium are 4.8 × 10-2 and 1.2 × 10-2 mol/L respectively. The value of Kc for the reaction is :
2NO2(g) ; the concentration of N2O4 and NO2 at equilibrium are 4.8 × 10-2 and 1.2 × 10-2 mol/L respectively. The value of Kc for the reaction is :
Matching List Type
Direction (Q, Nos. 18 and 19) Choices for the correct combination o f elements from Column I and Column II are given as options (a), (b), (c) and (d) out of which one is correct.
Q.
Match the Column ! with Column II and mark the correct option from the codes given below.
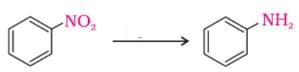
The following reaction takes place in the presence of
Direction (Q. Nos. 1-15) This section contains 11 multiple choice questions. Each question has four choices (a), (b), (c) and (d), out of which ONLY ONE option is correct.
Q. For the
I. benzene (C6H6) and
II. borazine (B3N3H6)
Select the correct statement.
Only One Option Correct Type
This section contains 11 multiple choice questions. Each question has four choices (a), (b), (c) and (d), out of which ONLY ONE is correct.
Q.
CsCI crystallises in a cube that has CP at each corner and Cs+ at the centre of the unit cell. If rCs+ = 169 pm and rcl- =181 pm, then edge length of the cube is
The rate equation for the reaction 2 A + B → C is found to be : rate = k [A] [B] . The correct statement in relation to this reaction is that the
[AIEEE-2004]
Only One Option Correct Type
This section contains 7 multiple choice questions. Each question has four choices (a), (b), (c) and (d), out of which ONLY ONE is correct
Q.
The degree of dissociation (α ) o f weak electrolyte AxBy is related to van’t Hoff factor (i) by the expression
[AIEEE 2011]
The enthalpy of neutralisation of HS- (aq) is - 5.1 kJ mol-1. Thus, second ionisation energy of H2S is
For macromolecules to form, one more of the following criteria are essential
An atom has a mass of 0.02 kg & uncertainity in its velocity is 9.218 × 10-6 m/s then uncertainity in position is (h = 6.626 × 10-34 J - s) [AIEEE- 2002]
The atom which defines the structure of a family of organic compounds and their properties is called ___________


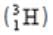 has 1 proton and 2 neutrons.
has 1 proton and 2 neutrons. 














