Bihar PGT Chemistry Mock Test - 2 - Bihar PGT/TGT/PRT MCQ
30 Questions MCQ Test - Bihar PGT Chemistry Mock Test - 2
The following sentence has been broken into four parts with an error in one part. Identify that part and mark it as your answer. If there are no errors in any of the given parts, mark option 4 or ‘No error’ as your answer.
Q. Rohan has neither spoken (1)/ nor (2)/ written to him. (3)/ No error (4).
Directions: In the following question, a sentence is given with a blank to be filled in with an appropriate word. Select the correct alternative out of the four and indicate it as your answer.
Kalidas, _______ wrote some fine dramas, is famous.
Kalidas, _______ wrote some fine dramas, is famous.
| 1 Crore+ students have signed up on EduRev. Have you? Download the App |
Directions: In the following question, out of the four alternatives, select the word opposite in meaning to the word given.
Castigate
In the given questions, fill in the blanks with the appropriate words from the alternatives provided.
Q. _________ contraction is a characteristic of certain groups of skeletal muscle.
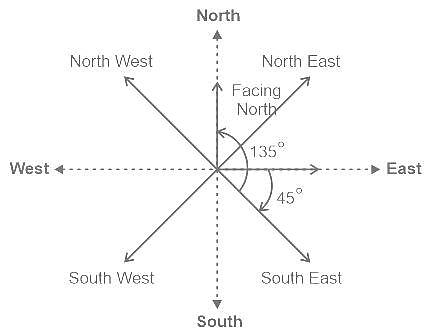
Sunny is facing East. After that, he turns 45° clockwise and then 135° anticlockwise. In which direction is he facing now?
Among the following, the one with the highest mass is:
Find the word that can be suffixed to the given words to form new meaningful words.
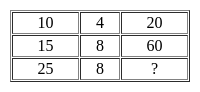
Fail, Press, Depart, AdventSelect the number which can be placed at the sign of the question mark (?) from the given alternatives.
The molal elevation constant is the elevation in boiling point of
A first order reaction is 50% completed in 1.26 × 1014 s. How much time would it take for 100% completion?
Entropy change when 2 moles of an ideal gas expands reversibly from an initial volume of 1 dm3 to a final volume of 10 dm3 at a constant temperature of 298 K is
Given, ΔfH° of HCI (g) is - 22 . 10 kcal mol-1 and ΔSolutionH° (heat of solution) of HCI (g) is - 17.9 kcal mol-1. Thus, ΔfH ° of Cl- (aq) is
If a is the degree of dissociation of Na2SO4, the vant Hoff's factor (i) used for calculating the molecular mass is–
[AIEEE-2005]
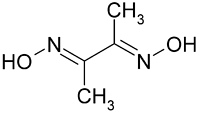
Which of the following represents chelating ligand?
Direction (Q. Nos. 1-18) This section contains 18 multiple choice questions. Each question has four
choices (a), (b), (c) and (d), out of which ONLY ONE option is correct.
Q.
The correct statement regarding a chiral compound is
Optical rotation of a newly synthesised chiral compound is found to be +60°. Which of the following experiment can be performed to establish that optical rotation is not actually -300°?
When sodium is dissolved in liquid ammonia, a solution of deep blue colour is obtained. The colour of the solution is due to
Be can show coordination number four while other members show a value of six. This is because of:
The decreasing order of the repulsive interactions between various electron pairs is: