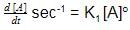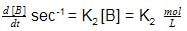Bihar PGT Chemistry Mock Test - 5 - Bihar PGT/TGT/PRT MCQ
30 Questions MCQ Test - Bihar PGT Chemistry Mock Test - 5
Directions: Improve the bracketed part of the sentence.
Q. He (like) to picture himself as an original thinker.
In the following question, out of the four alternatives, select the alternative which is the best substitute for the phrase.In the following question, out of the four alternatives, select the alternative which is the best substitute for the phrase.
Q. A man who is courteous and gallant in his behavior.
| 1 Crore+ students have signed up on EduRev. Have you? Download the App |
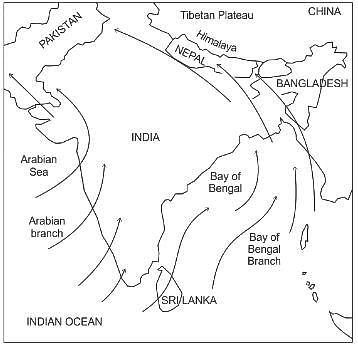
निम्नलिखित में से किस में पांच चौपाई के बाद एक दोहा देने की परंपरा नहीं है?
'भवन - भुवन' समश्रुत भिख्रार्थक श्वि का उचित अर्थ है -
Select the number which can be placed at the sign of the question mark (?) from the given alternatives.

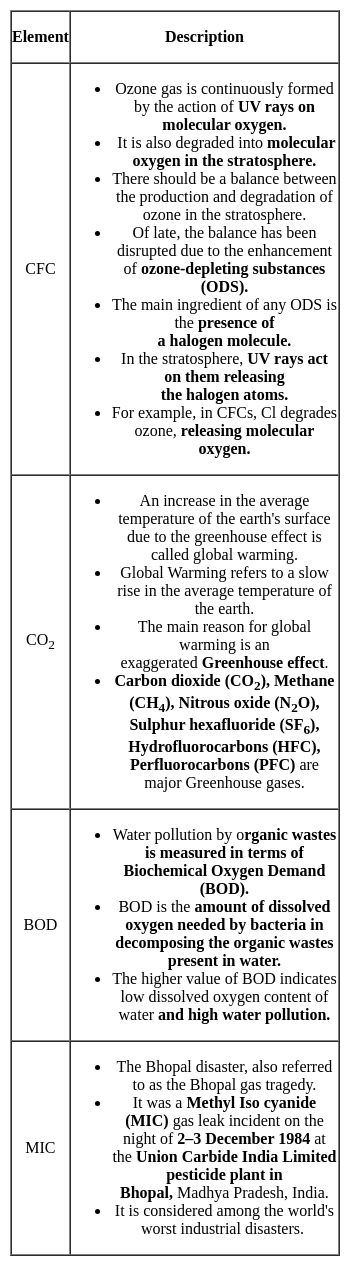
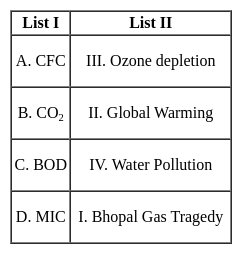
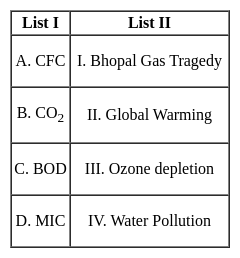
Match the items in List I with List II and select the correct answer using the codes given below:
Find the wrong number in the following number series:
121, 169, 289, 361, 529, 576
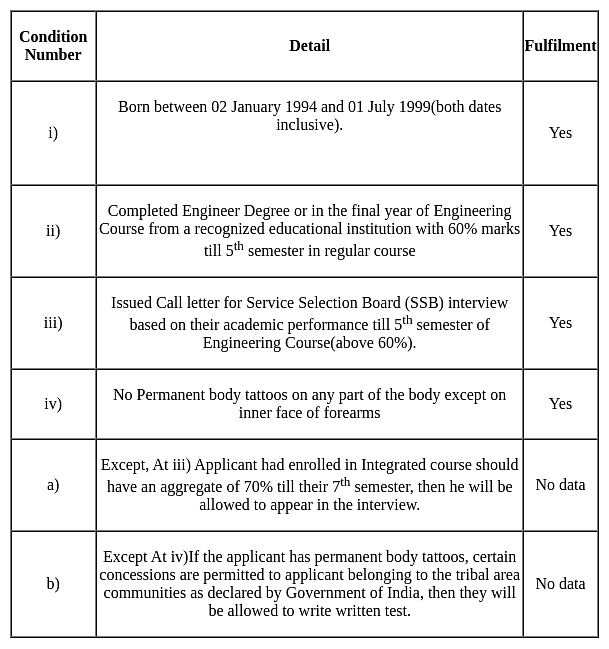
Abhijit born on 03 December 1995, completed his Engineering Degree with 70% marks till his 5th semester in a regular course, and he does not have any permanent body tattoos.
A metal in a compound can be displaced by another metal in the uncombined state. Which metal is a better reducing agent in such a case?
Consider following two reactions
Units of k1 and k2 are expressed in terms of molarity (mol L–1) and time (sec–1) as –
[AIEEE-2002]
Which of the following is not an example of redox reaction?
From the following, pick out the explanation for why molecule Y hydrolyses faster than molecule Z.
PCl5 dissociation a closed container as :
PCl5(g) 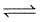 PCl3(g) + Cl2(g)
PCl3(g) + Cl2(g)
If total pressure at equilibrium of the reaction mixture is P and degree of dissociation of PCl5 is α, the partial pressure of PCl3 will be :
We have taken a saturated solution of AgBr.Ksp of AgBr is 12 x 10 – 14 . If 10 – 7 mole of AgNO3 are added to 1 litre of this solution then the conductivity of this solution in terms of 10 – 7 Sm – 1 units will be
[given 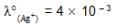 Sm2 mol-1
Sm2 mol-1 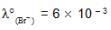 Sm2 mol-1, 5 x 10-3 Sm2mol-1]
Sm2 mol-1, 5 x 10-3 Sm2mol-1]
The quantum number which specifies the location of an electron as well as energy is
The number of radial nodes in 3s and 2p respectively are
Dinucleotide is obtained by joining two nucleotides together by phosphodiester linkage. Between which carbon atoms of pentose sugars of nucleotides are these linkages present?
One mole of each KMnO4 and K2Cr2O7 can oxidise ........... moles of ferrous ion.