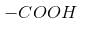HPSC PGT Chemistry Mock Test - 1 - HPSC TGT/PGT MCQ
30 Questions MCQ Test - HPSC PGT Chemistry Mock Test - 1
In which century Firoz Shah Tughlaq founded the city of Fatehabad?
A ruler of which dynasty gave a big part of Haryana a name, called 'Srikanth' janapada?
| 1 Crore+ students have signed up on EduRev. Have you? Download the App |
In a certain code, MONK is written as 53. How will TUTOR be written in that code?
Select the option which is related to the third term in the same way as the second term is related to the first term.
Synonym : Similar :: Opposite : ?
The following three aspects of intelligence are dealt with by Sternberg's triarchic theory except:
The major purpose of diagnostic test is that of identifying
Electronic configuration of the element having atomic number 24.
Which of the following is not the name of the 104th element?
Lothar Meyer proposed that on arranging the elements in order of increasing atomic weights; similarities appear in which type of properties?
Only One Option Correct Type
This section contains 10 multiple choice questions. Each question has four choices (a), (b), (c) and (d), out of which ONLY ONE is correct
Which of the following concentration factors is affected by change in temperature?
Only One Option Correct Type
Direction (Q. Nos. 1-8) This section contains 8 multiple choice questions. Each question has four choices (a), (b), (c) and (d), out of which ONLY ONE option is correct.
Q.
The incorrect statement concerning E1 reaction is
How many minimum no. of C-atoms are required for position & geometrical isomerism in alkene?
Which of the following concentration factor is affected by change in temperature ?
[AIEEE-2002]
BeSO4 is water soluble while CaSO4 is not. This is because of:
ΔfH° of CS2 = 117.36 kJ mol-1
C(g) = 716.682 kJ mol-1
S(g) = 278.805 kJ mol-1
Q. Thus, bond enthalpy in CS2 is
A current of 0.1A was passed for 2hr through a solution cuprocyanide and 0.3745g f copper was deposited on the cathode. Calculate the current efficiency for the copper deposition.
Direction (Q, Nos. 1 - 5) This section contains 5 multiple choice questions. Each question has four choices (a), (b), (c) and (d), out of which ONLY ONE option is correct.
Q. Which molecule will give the following dicarboxylic acid upon treatment with acidic solution of KMnO4?
Direction (Q. Nos. 1-15) This section contains 11 multiple choice questions. Each question has four choices (a), (b), (c) and (d), out of which ONLY ONE option is correct.
Q. For the
I. benzene (C6H6) and
II. borazine (B3N3H6)
Select the correct statement.
Direction (Q. Nos. 1 - 11) This section contains 11 multiple choice questions. Each question has four choices (a), (b), (c) and (d), out of which ONLY ONE option is correct.
Q. When light is shined on a mixture of chlorine and ethane, chloroethane is formed besides dichloroethane, trichloroethane and several other products. What reaction condition can optimise the yield of chloroethane?
Arrange the following compounds in decreasing order of their acid strength: i) trichloroacetic acid ii) trifluoroacetic acid iii) acetic acid and iv) formic acid
Among the following enthalpies, which is always less than zero?
The electronegativity of following elements increases in the order of?
The common features among the species CN-, CO, NO+ and N2 are
Direction (Q. Nos. 1-11) This section contains 11 multiple choice questions. Each question has four choices (a), (b), (c) and (d), out of which ONLY ONE option is correct.
Q. Calorific value of H2 is - 143 kJ g-1.Thus, ΔfH° of H2O is
Only One Option Correct Type
This section contains 7 multiple choice questions. Each question has four choices (a), (b), (c) and (d), out of which ONLY ONE is correct
Q.
The degree of dissociation (α ) o f weak electrolyte AxBy is related to van’t Hoff factor (i) by the expression
[AIEEE 2011]
How many moles of magnesium phosphate, Mg3 (PO4)2 will contain 0.25 mole of oxygen atoms?
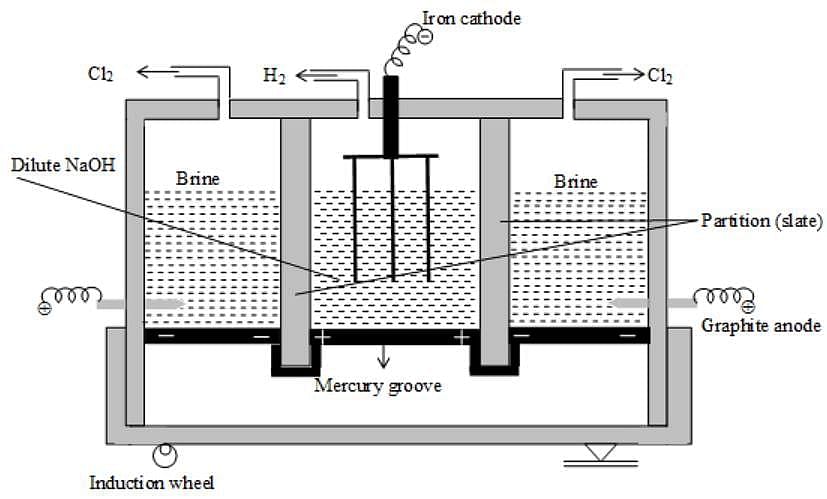
Which pair of electrolysis could be distinguished by the products of electrolysis using inert electrodes?
How many atoms of Oxygen are there in 18g of water?
(Hint: Avogadro’s Number = 6.02 x 1023 atoms/mol)
Direction (Q. Nos. 1-7) This section contains 7 multiple choice questions. Each question has four choices (a), (b), (c) and (d), out of which ONLY ONE is correct.
Q.
The hybridisation of N in solid state for N2O5 is





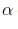 - position.
- position.