HPSC PGT Chemistry Mock Test - 2 - HPSC TGT/PGT MCQ
30 Questions MCQ Test - HPSC PGT Chemistry Mock Test - 2
Satyawart Kadian is associated with which of the following sports?
| 1 Crore+ students have signed up on EduRev. Have you? Download the App |
Recently who has been made the General Secretary of the Haryana Wrestling Association?
If one of the electrons (1s2) of helium is taken in excited state then bond order of He2 is
If X is a nonmetal, its oxide X2O3 is expected to be a/an ______ oxide.
For a hypothetical H like atom which follows Bohr's model, some spectral lines were observed as shown. If it is known that line 'E' belongs to the visible region, then the lines possibly belonging to ultra violet region will be (n1 is not necessarily ground state)
[Assume for this atom, no spectral series shows overlaps with other series in the emmission spectrum]
Correct decreasing order of dipole moment of CH3F, CH3CI and CH3Br is
PH3 becomes spontaneously inflammable due to the presence of
‘Hartree’ is the atomic unit of energy and is equal to
We can obtain ethylamine by Hoffmann bromamide reaction. The amide used in this reaction is:
An organic compound X (C5H12O) gives effervescence with NaH. X on treatment with acidic CrO3 solution turns solution to blue-green forming C5H10O2. Also X can be resolved into enantiomers. The correct statement regarding X is
Consider the following transformations.
Q. Which reaction sequence will bent bring about the above transformation?
For the reversible reaction,
In a reaction vessel, [NO]= [O2]= 0.01 mol L-1 and [NO2]= 0.1 mol L-1 then above reaction is
Out of NH3, H2O and HF, which has the highest magnitude of Hydrogen bonding:
Substances that alter the rate of a chemical reaction without being used up in a chemical reaction is known as
A chemical reaction [2A] + [2B] + [C] → product follows the rate equation : then order of reaction is - [AIEEE-2002]
When sodium is dissolved in liquid ammonia, a solution of deep blue colour is obtained. The colour of the solution is due to
Which of the following are all properties of nonmetals?
Be can show coordination number four while other members show a value of six. This is because of:
Which of the following has maximum boiling point?
Which of the following types of octahedral complexes will exhibit geometrical isomerism (where, M = metal, a, b = achiral ligands)?
What is the equilibrium constant for the reaction P4(s) + 5O2(g) 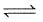 P4O10(s) :
P4O10(s) :
In the complete combustion of C2H6, 54 g of H2O is formed and 370 kcal of heat is evolved. Thus, ΔCH° of C2H6 is
Entropy change when 2 moles of an ideal gas expands reversibly from an initial volume of 1 dm3 to a final volume of 10 dm3 at a constant temperature of 298 K is
Maximum number of electrons in a subshell with l = 3 and n = 4 is
If a is the degree of dissociation of Na2SO4, the vant Hoff's factor (i) used for calculating the molecular mass is–
[AIEEE-2005]
How many grams of Mgl2 must be added to 250 mL of 0.0876 M Kl to produce a solution with [l-] = 0.10 M?