HPSC PGT Chemistry Mock Test - 5 - HPSC TGT/PGT MCQ
30 Questions MCQ Test - HPSC PGT Chemistry Mock Test - 5
Scurvy is caused by the deficiency of which vitamin?
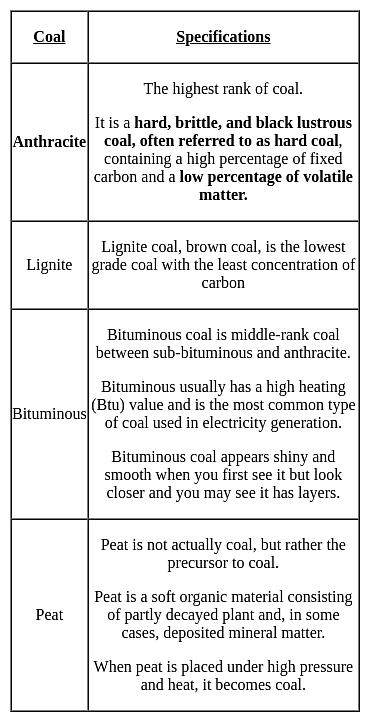
Which type of coal has the highest calorific value?
| 1 Crore+ students have signed up on EduRev. Have you? Download the App |
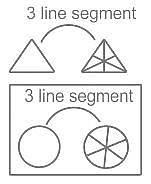
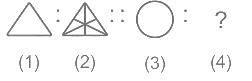
Select the figure that will come next in place of ?.

How does NEP 2020 address the issue of early dropouts from school?
What is the purpose of the National Repository of Educational Data (NRED) under NEP 2020?
In an extempore speech, the principal of a school asked the students to focus more on vocabulary. He opined that they must adopt the habit of learning the correct use of words. What according to you is the intelligence that is associated with the ability of students to use words effectively?
If pKb for fluoride ion at 25°C is 10.83, the ionisation constant of hydrofluoric acid in water at this temperature is :
The chemical reaction in which reactants require high amount of activation energy are generally
If 75% of a first order reaction was completed in 32 min, then 50% of the reaction was completed in
For the following three reactions 1, 2 and 3, equilibrium constants are given :
(1) CO(g) + H2O(g)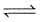 CO2(g)+H2(g) ; K1
CO2(g)+H2(g) ; K1
(2) CH4(g)+H2O(g)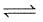 CO(g)+3H2(g) ; K2
CO(g)+3H2(g) ; K2
(3) CH4(g)+2H2O(g)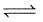 CO2(g)+4H2(g) ; K3
CO2(g)+4H2(g) ; K3
Which of the following relations is correct ?
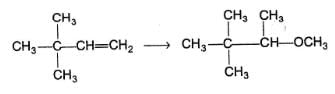
What is formed in the reaction below as major product?
Maximum number of compounds are known in case of :
Which is the most suitable reagent for the following transformation?
Many copper (I) compounds are unstable in aqueous solution and undergo disproportionation as 2Cu+ → Cu + Cu2+ . This is due to
Which of the following conclusions could not be derived from Rutherford’s α -particle scattering experiment?
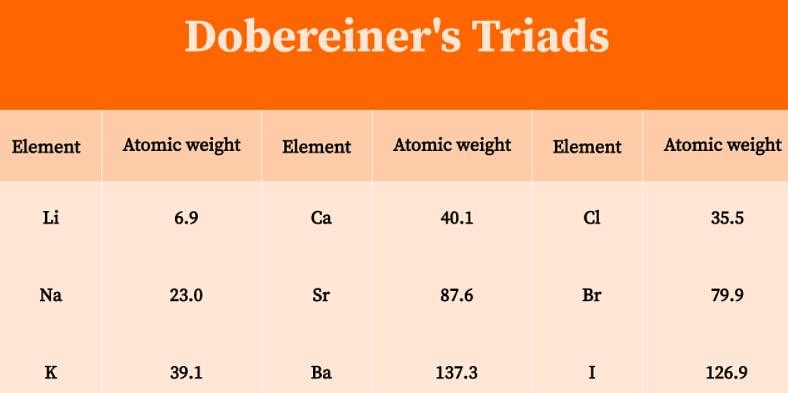
According to Dobereiner’s law of triads the number of elements present in each group is:
I− reduces IO3- and I2 and itself oxidised to I2 in acidic medium. Thus, final reaction is
The best set of reagents for the following transformation is
Dinucleotide is obtained by joining two nucleotides together by phosphodiester linkage. Between which carbon atoms of pentose sugars of nucleotides are these linkages present?
Direction (Q. Nos. 15-16) This section contains a paragraph, wach describing theory, experiments, data etc. three Questions related to paragraph have been given.Each question have only one correct answer among the four given options (a),(b),(c),(d)
The complex [Fe(H2O)5NO]2+ is formed in the ring-test for nitrate ion when freshly prepared FeSO4 solution is added to aqueous solution of
followed by the addition of conc. H2SO4. NO exists as NO+ (nitrosyl).
Q.Oxidation number of the Fe in the ring is
Which of the following statement is true about Langmuir’s adsorption?
Heat of hydrogenation of ethene is x1 and that of benzene is x2. The resonance energy of benzene is