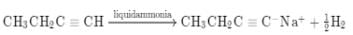HPSC PGT Chemistry Mock Test - 8 - HPSC TGT/PGT MCQ
30 Questions MCQ Test - HPSC PGT Chemistry Mock Test - 8
| 1 Crore+ students have signed up on EduRev. Have you? Download the App |
Who is the current secretary of Haryana Staff Selection Commission (HSSC)?
According to National Education Policy 2020, the progress card of the students communicated to the parents would be-
Lead emitted by vehicles interferes with development of:
Comprehension Type
This section contains a passage describing theory, experiments, data, etc. Two questions related to the paragraph have been given. Each question has only one correct answer out of the given 4 options (a), (b), (c) and (d)
Passage
A handbook gives the concentration of water in a 2.772 M aqueous solution of NaOH as 998.0 g L-1.
Q. Molality of solution is
Which of the following is NOT a molecular solid?
Oxidation number of 1/2 is assigned to oxygen atom in
In which of the following processes, does the value of magnetic moment change ?
Electron-rich hydrides has excess electrons that are present as
According to MO theory which of the following lists ranks the nitrogen species in terms of increasing bond order?
Consider the reactions
(A) H2O2 + 2HI → I2 +2H2O
(B) HOCl + H2O2 → H3O + Cl - - + O2
Which of the following statements is correct about H2O2 with reference to these reactions? Hydrogen perioxide is ________.
In an atom, an electron is moving with a speed of 600 m/s with an accuracy of 0.005%. Certainity with which the position of the electron can be located is (h = 6.6×10-34 kg m2s-1 ,mass of electron, em = 9.1×10-31 kg ) [AIEEE 2009]
The element which shows only negative oxidation state/s among following elements is:
In which of the following pairs both the ions are coloured in aqueous solution? (Atomic number, Sc = 21, Ti = 22, Ni = 28, Cu = 29, Co = 27)
The flow of current is possible in Daniel cell only
If x denoted the number of valence electrons of a representative element then its valence is equal to:
The hydrocarbon which can react with sodium in liquid ammonia is -
[AIEEE 2008]
A metal M readily forms water soluble sulphate, and water insoluble hydroxide M(OH)2. Its oxide MO is amphoteric, hard and having high melting point. The alkaline earth metal M must be -
In an atom, an electron is moving with a speed of 600m/s with an accuracy of 0.005%. Certainity with which the position of the electron can be located is (h = 6.6 ×10−34 Js)
The specific rotation of optically pure (R)-2- butanol is -13.52° at 25°C. An optically pure sample of (R)-2- bromobutane was treated with aqueous NaOH in order to form 2-butanol via SN2 reaction. What would be the specific rotation of the product assuming 100% yield?
Direction (Q. Nos. 17) Choice the correct combination of elements and column I and coloumn II are given as option (a), (b), (c) and (d), out of which ONE option is correct.
Match the compounds/ions having underlined atoms of different oxidation number (in Column I) with values (in Column II).
Direction (Q. Nos. 17-20) This section contains 4 multiple choice questions. Each question has four choices (a), (b), (c) and (d), out of which ONE or MORE THANT ONE is correct.
Q. Which of the following can be calculated based on Born-Haber cycle of formation of a lattice A+B- (s) from A(s) and 6 (g) ?
A coordination compound of cobalt has the molecular formula containing five ammonia molecules, one nitro group and two chlorine atoms for one cobalt atom. One mole of this compound produces three ions in an aqueous solution. The aqueous solution on treatment with an excess of AgNO3 gives two moles of AgCI as a precipitate. The formula of the complex and the isomerism shown by this is