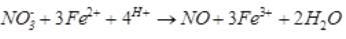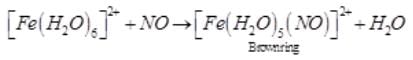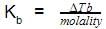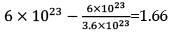HPSC PGT Chemistry Mock Test - 9 - HPSC TGT/PGT MCQ
30 Questions MCQ Test - HPSC PGT Chemistry Mock Test - 9
Which medal has recently been won by Haryana's Vinesh Phogat in the ongoing World Wrestling Championship in Belgrade?
| 1 Crore+ students have signed up on EduRev. Have you? Download the App |
To promote sustainable development Haryana developed a Forest Policy in the year
What is meant by 'nature' in 'nature-nurture' controversy?
In scientific notation for such numbers, any number can be represented in the form N × 10n where
A brown ring is formed in the ring test for NO3– ion. It is due to the formation of
The exothermic formation of ClF3 is represented by the equation :
Cl2(g) + 3F2(g)  2ClF3(g) ; ΔH = -329 kJ
2ClF3(g) ; ΔH = -329 kJ
Which of the following will increase the quantity of ClF3 in an equilibrium mixture of Cl2, F2 and ClF3 :
Comprehension Type
Direction (Q. Nos. 15-17) This section contains a passage describing theory, experiments, data, etc. Two questions related to the paragraph have been given. Each question has only one correct answer out of the given 4 options (a), (b), (c) and (d).
Passage
Oxirane is a reactive ether, undergo nucleophilic attack at a-carbon in the presence of both acidic and basic medium. The generalised mechanism in the two medium are :
I. Acidic medium
II. Basic medium
As shown above, in acidic medium, nucleophilic attack occur at more substituted α-carbon while in basic medium, nucleophilic attack occur preferably at less substituted α-carbon.
Q.
In the reaction given below, th e final major organic product Y is
Which compound given below has the highest solubility in water ?
What is the electronic configuration of carbon in it’s excited state?
The maximum number of atomic orbitals associated with a principal quantum number 5 is
What compound is produced when (CH3)2CHCH2Br is subjected to the following sequence of steps:
1. Mg, Et2O
2. CO2
3. H3O+?
Comprehension Type
Direction (Q. Nos. 13-15) This section contains a passage describing theory, experiments, data, etc. Two questions related to the paragraph have been given. Each question has only one correct answer out of the given 4 options (a), (b), (c) and (d).
Passage
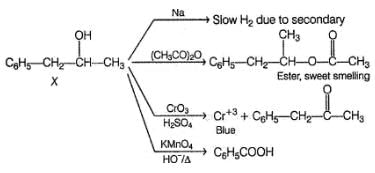
An organic compound X (C9H12O) gives the following reactions :
i. Na - Slow gas bubble formation
ii. Acetic anhydride - Pleasent smelling liquid
iii. CrO3-H2SO4 - Blue-green solution
iv. Hot KMnO4 - Benzoic acid
v. Br2-CCI4 - No decolouration
vi. I2 + NaOH - Yellow solid is formed
vii. X rotates the plane polarised light
Q.
The structure of X is
What is the correct structure for the major compound produced by the following reaction sequence?
Number of electrons having l + m value equal to zero in 26Fe may be
The phenomenon in which adsorption and absorption takes place simultaneously is called?
Rate of reaction for the combustion of propane is equal to
C3H8(g) + 5O2(g) → 3CO2(g) + 4H2O(g)
For Cr(24), (EC) : [Ar] 4s1 3d5 The number of electrons with l = 1 to l = 2 are respectively
Direction (Q. Nos. 11-14) This section contains 4 multiple choice questions. Each question has four choices (a), (b), (c) and (d), out of which ONE or MORE THANT ONE is correct.
Q. For an ideal gas, consider only (p -V) work in going from initial state X to the final state Z. The final state Z can be reached either of the two paths shown in the figure. Which of the following choice (s) is (are) correct?
(Take ΔS as change in entropy and W as work done)
[IIT JEE 2012]
Reaction
+ HOCl → product,
here product will be -
[AIEEE-2002]
Comprehension Type
Direction (Q. Nos. 15-17) This section contains a paragraph, describing theory, experiments, data, etc. Three questions related to the paragraph have been given. Each question has only one correct answer among the four given options (a), (b), (c) and (d).
Passage
Glycol when heated with concentrated H2SO4, undergo a variety of reactions as

Nature of predominant product depends upon the nature of other groups present at the two α-carbons.
Q.
Which is most likely to undergo intermolecular dehydration to give dioxane or substituted dioxane?
The molal elevation constant is the ratio of the elevation in B.P to:
Newland arranged elements in increasing order of atomic weights and noted that every eighth element had properties similar to:
How much time (in hours) would it take to distribute one Avogadro number of wheat grains if 1020 grains are distributed each second?
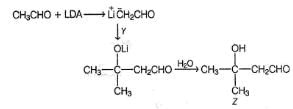
Consider the following reaction,
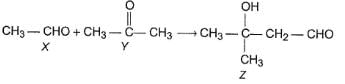
Q.
How the product Z can be prepared selectively using X and Y and other reagents?
NO2 can be represented as
Q. Formal charge on each oxygen atom is