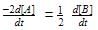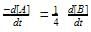HPSC PGT Chemistry Mock Test - 10 - HPSC TGT/PGT MCQ
30 Questions MCQ Test - HPSC PGT Chemistry Mock Test - 10
Which famous folk dance of Haryana is performed by both Men & Women?
| 1 Crore+ students have signed up on EduRev. Have you? Download the App |
In which of the following month Pinjore heritage festival is celebrated in Haryana?
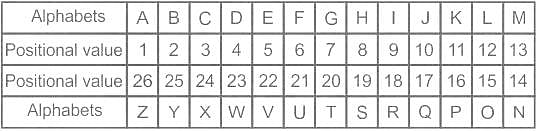
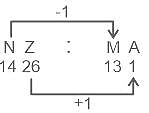
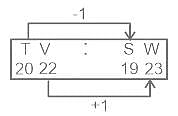
In the following question, select the related letters from the given alternatives.
NZ : MA :: TV : ?
Heating limestone at a temperature of 1070 K we get:
Alkaline earth metals combine with halogens to form:
Direction (Q. Nos. 1-18) This section contains 18 multiple choice questions. Each question has four
choices (a), (b), (c) and (d), out of which ONLY ONE option is correct.
Q. Oxidation can be defined as the terms
I. gain of electron and hydrogen
II. gain of oxygen and loss of electron
III. increase in oxidation number
IV. decrease in oxidation number
Select the correct terms
For a reaction 1/2 A→ 2B, rate of disappearance of ‘A’ related to the rate of appearance of ‘B’ by the expression -
[AIEEE 2008]
The conversion of primary aromatic amines into diazonium salts is known as:
Among the following compounds the maximum number of lone pair is present on the central atom of:
Direction (Q. Nos. 1- 8) This section contains 8 multiple choice questions. Each question has four choices (a), (b), (c) and (d), out of which ONLY ONE option is correct.
Q. For the given reaction, = - 1.3818 kcal at 300 K. Thus equilibrium constant is
amongst the following compounds, the optically active alkane having lowest molar mass is
[AIEEE 2004]
In a first order reaction, the concentration of the reactant, decreases from 0.8 M to 0.4 M in 15 minutes. The time taken for the concentration to change from 0.1 M to 0.025 M is -
[AIEEE-2004]
Requirement of macronutrient per acre of the land is
Passage
Consider the following isomers of [Co(NH3)4Br2]+. The black sphere represents Co, grey sphere represents NH3 and unshaded sphere represents Br.
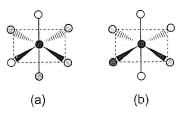
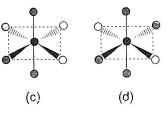
Q.
Which of the structures is identical?
Direction (Q. Nos. 14 -15) This section contains a paragraph, wach describing theory, experiments, data etc. three Questions related to paragraph have been given.Each question have only one correct answer among the four given ptions (a),(b),(c),(d).
Based on the following thermochemical data of the given process, answer the questions.
Q. Bond energy of (C— C) bond is
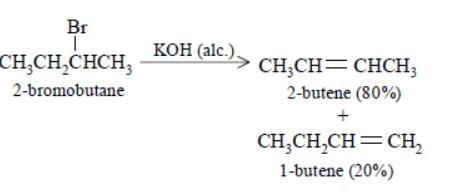
Elimination of bromine from 2–bromobutane results in the formation of –
[AIEEE-2005]
C7H7Cl shows how many benzenoid aromatic isomers?
In the following set of nucleophiles, the strongest and the weakest nucleophile respectively are
I. CH3S-
II. CH3COC-
III. HO-
IV. C6H5C-
In which of the following cases, entropy of I is larger than that of II?
A red solid is insoluble in water. However, it becomes soluble if some KI is added to water. Heating the red solid in a test tube results in liberation of some violet coloured fumes and droplets of a metal appear on the cooler parts of the test tube. The red solid is
What compound is produced when cyclohexene is treated with concentrated KMnO4?
Passage lI
One of the reactions that takes place in producing steel from iron ore is the reduction of iron (II) oxide by carbon monoxide to give iron metal and carbon dioxide.
Initial partial pressure
CO(g) = 1.40 atm
CO2(g) = 0.80 atm
Q. Under the given partial pressure, reaction is


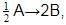 rate of disappearance of A is related to rate of appearance of B by the expression:
rate of disappearance of A is related to rate of appearance of B by the expression: