AWES PGT Chemistry Mock Test - 1 - AWES TGT/PGT MCQ
30 Questions MCQ Test - AWES PGT Chemistry Mock Test - 1
Who has been appointed to lead Microsoft's Windows and Surface teams?
| 1 Crore+ students have signed up on EduRev. Have you? Download the App |
You are a class teacher of the seventh standard. A concerned mother consults you on the changing behaviour of her daughter and her continuous loss of interest in her studies. You hold the stage and her involvement in her peer groups to be responsible for this. The stage that marks the involvement of children in their peer groups is
In an experiment conducted on a hungry chimpanzee, some bananas were kept outside its cage, but beyond its reach. Some sticks were also kept in its cage. After several unsuccessful attempts to reach out to the bananas, the chimpanzee pondered over the problem. Then, he picked up a stick and pulled the bananas towards itself. In this case, learning took place by
Which of the following is not the name of the 104th element?
Consider the following statements, "According the Werner's theory. :
(1) Ligands are connected to the metal ions by covalent bonds.
(2) Secondary valencies have directional properties.
(3) Secondary valencies are non-ionisable.
(4) Secondary valencies are satisfied by either neutral or negative legands.
Of these statements.
Only One Option Correct Type
This section contains 11 multiple choice questions. Each question has four choices (a), (b), (c) and (d), out of which ONLY ONE is correct.
Q.
CsCI crystallises in a cube that has CP at each corner and Cs+ at the centre of the unit cell. If rCs+ = 169 pm and rcl- =181 pm, then edge length of the cube is
Out of the following, amphiprotic species are
I : HPO32-
II OH-
III H2PO4-
IV HCO3-
Which of the following can be termed as mixed complex?
ΔHvap = 30 kJ mol-1 and ΔSvap = 75 J mol-1 K-1. Thus, temperature of vapour at one atmosphere is
[IIT JEE 2004]
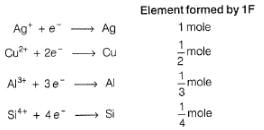
1 Faraday of electricity is passed through the solution containing 1 mole each of AgNO3, CuSO4, AlCl3 and SiCl4. Elements are discharged at the cathode.Number of moles of Ag, Cu,Al and Si formed will be in the ratio of
BeSO4 is water soluble while CaSO4 is not. This is because of:
In the structure of NaCI given below, ratio rNa+/rcl- is
Due to the presence of electrons in the inner shells, the electron in the outer shell will not experience the full positive charge of the nucleus (Ze). This is known as
How many moles of magnesium phosphate, Mg3 (PO4)2 will contain 0.25 mole of oxygen atoms?
The enthalpy of neutralisation of HS- (aq) is - 5.1 kJ mol-1. Thus, second ionisation energy of H2S is
Which property of colloids is applied in rubber plating & sewage disposal?
Only One Option Correct Type
This section contains 16 multiple choice questions. Each question has four choices (a), (b), (c) and (d), out of which ONLY ONE is correct
Q.
During the electrolysis of aqueous Zn(NO3)2 solution
For an endothermic reaction, the minimum value for the energy of activation in terms of will be
An atom has a mass of 0.02 kg & uncertainity in its velocity is 9.218 × 10-6 m/s then uncertainity in position is (h = 6.626 × 10-34 J - s) [AIEEE- 2002]
Which one of the following statement is false?
[AIEEE-2004]
In a 0.2 molal aqueous solution of a weak acid (HX), depression in freezing point is 0.383° Kf is 1.86° mol-1 kg. Assum e molarity equal to molality.
Match the parameters in Column I with their values in Column II and select the answer from the codes given below.
Cs+ ions impart violet to Bunsen flame. This is due to the fact that the emitted radiations are of
Which among the following substances is an example of multimolecular colloids?
A ‘100 proof solution of ethanol in water consists of 50.0 mL of C2H5OH(/)and 50.0 mL of H20 (/), mixed at 16 °C . Given,
Density of H20 = 1 g mL-1
Density of C2H,OH = 0.7939 gmL-1
Density mixture = 0.9344 g mL-1
Volume of the solution is
Direction (Q. Nos. 1-8) This section contains 8 multiple choice questions. Each question has four choices (a), (b), (c) and (d), out of which ONLY ONE is correct.
Q,
Which of the following pairs of compounds can be used as starting material in the synthesis of 2-phenyl-2-pentanol?
Propanamide on treatment with bromine in an aqueous solution of sodium hydroxide gives:
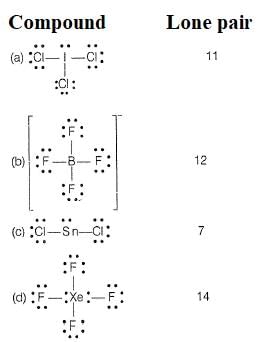
Which of the following molecule/species has the minimum number of lone pairs?