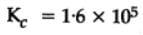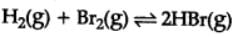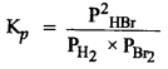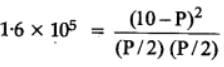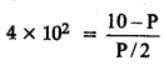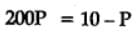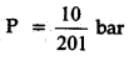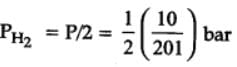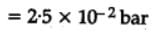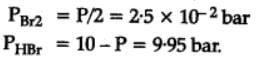AWES PGT Chemistry Mock Test - 3 - AWES TGT/PGT MCQ
30 Questions MCQ Test - AWES PGT Chemistry Mock Test - 3
With whom did India sign a Memorandum of Understanding (MoU) regarding semiconductors to enhance the supply chain and align with global developments?
| 1 Crore+ students have signed up on EduRev. Have you? Download the App |
Which Union Ministry launched the ‘ULLAS (Understanding Lifelong Learning for All in Society) initiative’?
Which institution partnered with Starburst to boost India's Aerospace, New Space, and Defence (ASD) startup ecosystem?
Which country's navy participated in the Varuna-23 naval exercise with the Indian Navy in Phase-II?
How are monthly or quarterly seminars by students helpful in senior classes?
Which one of the following is a form of Sternberg's triarchic theory of intelligence?
The UN organization that is related with education is
Under NEP 2020, what is the target for the Gross Enrollment Ratio (GER) in higher education by 2035?
Which of the following compounds will exhibit cis-trans isomerism?
Wave number of a spectral line for a given transition is x cm-1 for He+, then its value for Be3+ (isoelectronic of He+)for the same transition is
Stability of (C—Cl) bond in chlorobenzene is sim ilar to (C—Cl) bond in
At 87°C, the following equilibrium is established
H2(g) + S(s) 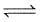 H2S(g) Kp = 7 × 10-2
H2S(g) Kp = 7 × 10-2
If 0.50 mole of hydrogen and 1.0 mole of sulfur are heated to 87°C in 1.0 L vessel, what will be the partial pressure of H2S at equilibrium ?
The equilibrium constant for the following reaction, is 1.6 x 105 at 1024 K.
H2(g) + Br2(g) ⇌2HBr(g)
HBr (g)at 10.0 bar is introduced into a sealed container at 1024 K. Thus, partial pressure of H2(g)and Br2(g), together is
Glucose solution is one molal. Glucose present in 1 kg glucose solution is
A hydrocarbon X is optically. X upon hydrogenation gives an optically inactive alkane Y. Which of the following pair of compounds can be X and Y respectively?
A resistance of 50Ω is registered when two electrodes are suspended into a beaker containing a dilute solution of a strong electrolyte such that exactly half of the them are submerged into solution. If the solution is diluted by adding pure water (negligible conductivity) so as to just completely submerge the electrodes, the new resistance offered by the solution would be
Silver metal crystallises in a cubic closest packed arrangement with edge length 407 pm. Thus, radius of the silver atom is
3 moles of a diatomic gas are heated from 127° C to 727° C at a constant pressure of 1 atm. Entropy change is (log 2.5 = 0 .4)
One or More than One Options Correct Type
This section contains 5 multiple type questions. Each question has 4 choices (a), (b), (c) and (d), out of which ONE or MORE THAN ONE are correct.
Q.
In hexagonal close packing,
Bond dissociation energy of = + 590 kJ mol-1 and that of (C—C) = + 331 kJ mol-1 at 298 K.
Enthalpy change for the polymerisation of ethene to polyethene is (where n is large integral value)
Temporary hardness It can be removed in boiling by precipitating
Select the set of compounds with oxidation-reduction duality.
The golden yellow colour associated with NaCl to Bunsen flame can be explained on the basis of -