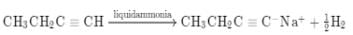AWES PGT Chemistry Mock Test - 8 - AWES TGT/PGT MCQ
30 Questions MCQ Test - AWES PGT Chemistry Mock Test - 8
Exercise Cutlass Express 2024 was organized in which country?
| 1 Crore+ students have signed up on EduRev. Have you? Download the App |
In which of the following processes, does the value of magnetic moment change ?
One or More than One Options Correct Type
This section contains 5 multiple type questions. Each question has 4 choices (a), (b), (c) and (d), out of which ONE or MORE THAN ONE are correct.
Select the correct point(s) of distinction between a volatic cell and electrolysis cell.
25.3 g of sodium carbonate, Na2CO3 is dissolved in enough water to make 250 mL of solution. If sodium carbonate dissociates completely, molar concentration of sodium ions, Na+ and carbonate ions, 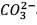 are respectively. (At. mass of carbon = 40)
are respectively. (At. mass of carbon = 40)
Type of isomerism exhibited by [Cr(NCS)(NH3)5] [ZnCl4] :
The element which shows only negative oxidation state/s among following elements is:
What is the major product in the following reaction?
Which of the following metal solution cannot be prepared by Bredig’s arc method?
The hydrocarbon which can react with sodium in liquid ammonia is -
[AIEEE 2008]
In Dobereiner's Triads, elements were grouped based on their similar chemical properties. Which of the following elements was not part of any known Dobereiner's Triad?
A metal M readily forms water soluble sulphate, and water insoluble hydroxide M(OH)2. Its oxide MO is amphoteric, hard and having high melting point. The alkaline earth metal M must be -
A group of 14 element is converted into n – type semiconductor by dopping it with
Which among the following is an instantaneous reaction?
The element having highest ionisation potential is:
For the cell (at 298 K)
Ag(s) | AgCl(s) | Cl-(aq) || AgNO3(aq) | Ag(s)
Which of the following is correct –
Which of the following sets of quantum numbers is correct for an electron in 4f orbital ? [AIEEE- 2004]
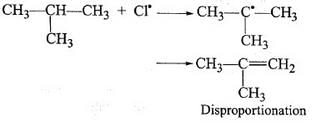
During free radical bromination of isobutane, an alkene is produced as by product via disproportionation of the intermediate alkyl free radical. What is this alkene?
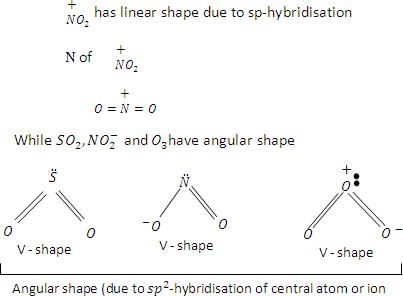
Among the following species linear shape is found in:
Which of the following is isoelectronic with F–?
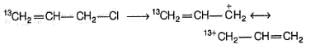
Statement I : When 3-bromo propene, which contain a labelled 13C at C-1 position is refluxed with methanol, following products were obtained.
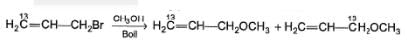
Statement II : Methanol has an acidic proton bonded to oxygen.


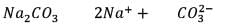
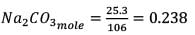
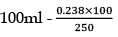
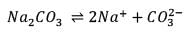
 0.955
0.955