AWES PGT Chemistry Mock Test - 9 - AWES TGT/PGT MCQ
30 Questions MCQ Test - AWES PGT Chemistry Mock Test - 9
What is the affirmed rating for State Bank of India (SBI) and Canara Bank by Fitch Ratings?
On what date was World Soil Day formally established as a global awareness-raising platform by the UN General Assembly?
| 1 Crore+ students have signed up on EduRev. Have you? Download the App |
Buzi Bridge, which was inaugurated recently, was built by India in which country?
Which Union Ministry is associated with the Cinematograph (Amendment) Bill, 2023?
In the Global Remote Work Index (GRWI), what is India's ranking among 108 countries?
In a progressive set-up children with special needs:
Johnson is an experienced teacher. He came to the class yesterday and told his students that they have reached the stage at which they are capable of thinking rationally about events and objects. Which stage is Johnson referring to at which a child starts thinking logically about events and objects?
Which among the following are the components of reciprocal teaching activity?
Ankit, a 12 years boy, is not enrolled in a school due to some circumstances. He has requisite computational skills and intends to enrol in a school to learn. Ankit has an
In the catalyzed decomposition of benzene diazonium chloride,
Half life period is found to be independent of the initial concentration of the reactant. After 10 min, the volume of N2 gas collected is 10 L and after the reaction is complete, it is 50 L. Hence, the rate constant of the reaction(in min-1) is
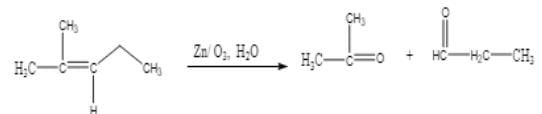
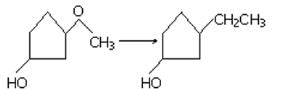
Ozonolysis of an organic compound ‘A’ produces acetone and propionaldehyde in equimolar mixture. Identify ‘A’ from the following compounds :
[AIEEE 2011]
The energy required to break one mole of Cl - Cl bonds in Cl2 is 242 kJ mol-1. The longest wavelength of light capable of breaking a single Cl - Cl bond is(c = 3 x 108 ms-1 and NA = 6.02 x 1023 mol-1) [AIEEE 2010]
Consider the cell Ag(s)|AgBr(s)|Br-(aq)||AgCl(s)|Cl-(aq)|Ag(s) at 25°C. The solubility product constants of AgBr & AgCl are respectively 5 X 10 – 13 & 1 X 10 – 10. For what ratio of the concentrations of Br- & Cl- ions would the emf of the cell be zero ?
The atomic radii of Cu and Ag are 1.17 Å and 1.34 Å. The atomic radius of gold is expected to be
Arrange the following in increasing order of reactivity in an SN2 reaction with K I in acetone solvent.
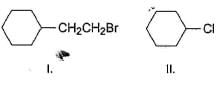
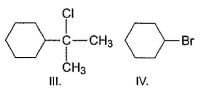
Aniline does not undergo Friedel – Crafts reaction
Compound of metal ion MX+(Z = 26) has a spin only magnetic moment of BM The number of unpaired electrons and x value in the compound are
Which of the following carbohydrate is an example of an oligosaccharide?
Which one of the following shows oxidation state upto + 7?
Which of the following has highest boiling point?
Which of the following is not a use of hydrogen peroxide?
Which is the correct statem ent about structure?
Which among the following is an example of first order reaction?
If a reaction proceeds with a uniform rate throughout, the reaction is
Among the following species the pair that have V-shaped geometry is:


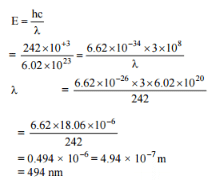
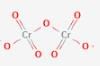
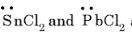 are bent shape molecule.
are bent shape molecule.














