AWES PGT Chemistry Mock Test - 10 - AWES TGT/PGT MCQ
30 Questions MCQ Test - AWES PGT Chemistry Mock Test - 10
| 1 Crore+ students have signed up on EduRev. Have you? Download the App |
When is World Down Syndrome Day observed annually?
Which bank announced a $250 million lending support for start-ups and 'new economy' companies in India?
Which case is famous for the definition of basic structure of the Indian Constitution?
In Lyman series, shortest wavelength of H-atom appears at x m, then longest wavelength in Balmer series of He+ appear at
Out of Cr (VI) as
, which is better oxidising agent?
In the following set of nucleophiles, the strongest and the weakest nucleophile respectively are
I. CH3S-
II. CH3COC-
III. HO-
IV. C6H5C-
Direction (Q. Nos. 13 and 14) This section contains a paragraph, describing theory, experiments, data, etc. Two questions related to the paragraph have been given. Each question has only one correct answer among the four given options (a), (b), (c) and (d).
Passage
In the all oxyacids of phosphorus, each phosphorus atom is in sp3-hybridised state. All these acids contain P—OH bonds, the hydrogen atom of which are ionisable imparting acidic nature to the compound. The ‘ous’ acids (oxidation state of P is + 1 or + 3) also have P—H bonds in which hydrogens are not ionisable.
The presence of P—H bonds in these acids imparts reducing properties. The structure of some oxyacids are drawn below:
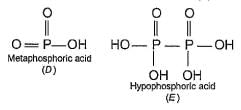
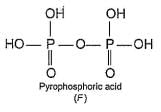
Q.
Although metaphosphoric acid is written as a monomer, it exists as a polymer (HPO3)n. The number of P—O—P bonds in cyclic trimetaphosphoric acid is
Water has a maximum density at _____ degree centigrade.
When Al2O3 is electrolysed ,cation and anions are discharged. For a given quantity of electricity,ratio of number of moles of Al and O2 gas is
A red solid is insoluble in water. However, it becomes soluble if some KI is added to water. Heating the red solid in a test tube results in liberation of some violet coloured fumes and droplets of a metal appear on the cooler parts of the test tube. The red solid is
Among the following elements, which one was initially placed in the 'rare earths' group in Mendeleev's Periodic Table?
What compound is produced when cyclohexene is treated with concentrated KMnO4?
Which of the ligand can show linkage isomerism and acts as flexidentate ligand:
The maximum number of electrons that can have principal quantum number, n = 3 and spin quantum number,
For the following equilibrium starting with 2 moles SO2 and 1 mole O2 in 1 L flask,
Equilibrium mixture required 0.4 mole in acidic medium. Hence, Kc is
A fully charged battery contains 500 mL of 5.00 M H2SO4. What is the concentration of H2SO4 in the battery after 6.0 A of current is drawn from the battery for 13.40 h?
Which type of a property is the Brownian movement of colloidal solution?
Match the species in Column I with the shape in Column II.
Which among the following is an example of photochemistry used in our daily life?
We have
I. 25 mL of 1 M NaOH
II. 10 mL of 0.50 M NaCI
On mixing the two solutions, molar concentrations of Na+, OH- and Cl- respectively, are
What is the dominant intermolecular force or bond that must be overcome in converting liquid CH3CH2OH to vapours?
Which one of the following sets of ions represents the collection of isoelectronic species ? [AIEEE- 2004]
An organic compound X has molecular formula C11H16O and it can be resolved into enantiom ers. X does not evolve any gas with Na metal. X when treated with concentrated HBr gives C6H5OH and Y(C5H11Br). Y is chiral and has the same configuration as that of compound X.
Q.
If Y is treated with C2H5ONa/C2H5OH then how many different E2 products would be formed?
Passage II
For oxidation of iron at 298 K, 4 Fe (s) + 3 O2(g) → 2 Fe2O3(s)
ΔS° = - 549.4 JK-1 mol-1 and ΔH ° = - 1648 . 0 kJ mol-1
Q. Free energy change for this reaction is
Direction (Q. No. 10) This sectionis based on statement I and Statement II. Select the correct answer from the code given below.
Q.
Statement I : Based on the following thermodynamic data
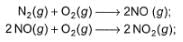
NO2 is more stable than NO.
Statement II : NO (g) is an endothermic compound while, NO2(g) is an exothermic compound.


















